October 28, 2020
New 3D cell culture method points to personalized cancer therapies
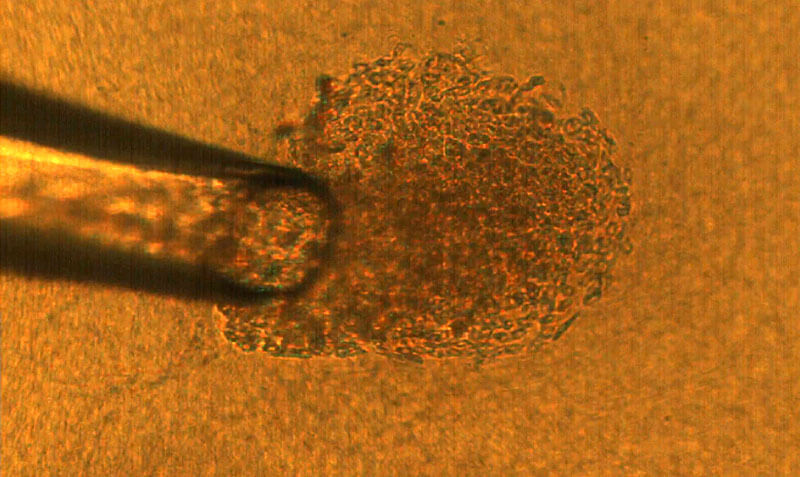 A 50-micron glass pipette is used to capture a single cancer cell, which is then deposited onto a matrix gel island to culture into a three-dimensional tumor. That tumor can be tested under laboratory conditions as an analog for the same tumor in a human body. (Purdue University image/Rohil Jain)
A 50-micron glass pipette is used to capture a single cancer cell, which is then deposited onto a matrix gel island to culture into a three-dimensional tumor. That tumor can be tested under laboratory conditions as an analog for the same tumor in a human body. (Purdue University image/Rohil Jain)
Technique recreates tumors in the lab from single cells
WEST LAFAYETTE, Ind. — Each cancer patient’s tumors have cells that look and act differently, making it difficult for scientists to determine treatments based on tumors grown from generic cell cultures in the lab.
Now, thanks to a new 3D cell culture technique developed by Purdue University researchers, it may be possible to personalize treatment by understanding the contributions of different cell types in a tumor to the cancer’s behavior.
“I see a future where a cancer patient gives a blood sample, we retrieve individual tumor cells from that blood sample, and from those cells create tumors in the lab and test drugs on them,” said Cagri Savran, a Purdue professor of mechanical engineering. “These cells are particularly dangerous since they were able to leave the tumor site and resist the immune system.”
Cell culture is a technique that biologists use to conduct research on normal tissue growth as well as on specific diseases. A 3D cell culture permits the formation of tumors from cancer cells that grow in three dimensions, meaning that the tumor is more like a three-dimensional potato than a two-dimensional leaf.
The Purdue team is the first to demonstrate a 3D cell culture from individually selected cells. This feat, described in a paper published in Scientific Reports, would allow scientists to more accurately know the impact of each cell on a tumor’s formation and behavior.
“To produce tissue samples that are close to what we have in the body, which allows us to do high-fidelity research in the laboratory, we need to place cells in an environment that mimics their natural milieu, allowing the cells to organize into recognizable structures like tissues in vivo,” said Sophie Lelièvre, a professor of cancer pharmacology in Purdue’s College of Veterinary Medicine.
Current 3D cell culture techniques have their limits, said Lelièvre, who studies 3D cell culture and helps design new cell culture methods in her role as scientific director of the 3D Cell Culture Core (3D3C) Facility at the Birck Nanotechnology Center of Purdue’s Discovery Park.
Real tumors, for example, are made up of cells of various phenotypes, or behaviors. How different these cells are from each other is described by the term “heterogeneity.”
The cellular heterogeneity of real tumors is not fully understood.
“Within a tumor, most cells are cancerous, but they do not have the same phenotype,” Lelièvre said. “It has been proposed that some tumors respond to chemotherapy, and some are resistant depending on the degree of heterogeneity of these phenotypes. It’s difficult to pinpoint treatments based on tumors grown in the lab because every patient’s tumors have different levels of heterogeneity.”
A typical cell culture dish or device also has a large number of cells. Scientists have no control over which cells develop into tumors. To understand how the heterogeneity inside a tumor develops and drives resistance to treatment, scientists need to study the contribution of each cell phenotype to the tumor by selecting individual cells and studying their impact.
Savran had previously demonstrated a microfluidic device capable of isolating single cancer cells from a blood sample.
“These cells are extremely rare,” Savran said. “With a sample with billions of cells, we may find just one or two tumor cells. But since we’ve figured out how to find them, we can now hand them off to people like Sophie to help study their heterogeneity.”
Savran’s team created a mechanical device that successfully extracted single tumor cells from existing cell lines of breast and colon cancers. They deposited each single cell onto a matrix gel island following Lelièvre’s advice.
After several days, the team observed that many of the selected single cells had developed into tumors that displayed degrees of aggressiveness corresponding to the cancer subtype of origin. The cells also recreated phenotypic heterogeneity, as shown with an imaging-based quantitative approach used previously by the Lelièvre lab.
“What Cagri’s technique did is really priceless,” Lelièvre said. “By simply analyzing the morphology of the tumors developed from individual cells, we could confirm that the degree of heterogeneity among tumors of the same cancer subtype increases with time without any other pressure or stimuli than those exerted by the growth of the tumor itself.”
The researchers also demonstrated that the degree of phenotypic heterogeneity inside a tumor depends on the cell of origin and could be related to fast-growing tumors for a specific breast cancer subtype, bringing new directions of research to understand the underlying mechanisms of aggressiveness in cancers.
“Creating specific treatments that can address an individual patient’s cancer is the Holy Grail of personalized therapy, and now we’re one step closer,” Savran said.
The Purdue Research Foundation Office of Commercialization has filed a patent on this technology. The work was supported by the National Science Foundation (Award 1509097) and the Tom Hurvis and the McKinley Educational Foundation. The Purdue Center for Cancer Research supported publication costs.
About Discovery Park
Discovery Park is a place where Purdue researchers move beyond traditional boundaries, collaborating across disciplines and with policymakers and business leaders to create solutions for a better world. Grand challenges of global health, global conflict and security, and those that lie at the nexus of sustainable energy, world food supply, water and the environment are the focus of researchers in Discovery Park. The translation of discovery to impact is integrated into the fabric of Discovery Park through entrepreneurship programs and partnerships.
About Purdue University
Purdue University is a top public research institution developing practical solutions to today’s toughest challenges. Ranked the No. 5 Most Innovative University in the United States by U.S. News & World Report, Purdue delivers world-changing research and out-of-this-world discovery. Committed to hands-on and online, real-world learning, Purdue offers a transformative education to all. Committed to affordability and accessibility, Purdue has frozen tuition and most fees at 2012-13 levels, enabling more students than ever to graduate debt-free. See how Purdue never stops in the persistent pursuit of the next giant leap at https://purdue.edu/.
Media contact: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Writer: Jared Pike
Sources: Sophie Lelièvre, lelievre@purdue.edu
Cagri Savran, savran@purdue.edu
Journalists visiting campus: Journalists should follow Protect Purdue protocols and the following guidelines:
- Campus is open, but the number of people in spaces may be limited. We will be as accommodating as possible, but you may be asked to step out or report from another location.
- To enable access, particularly to campus buildings, we recommend you contact the Purdue News Service media contact listed on the release to let them know the nature of the visit and where you will be visiting. A News Service representative can facilitate safe access and may escort you on campus.
- Correctly wear face masks inside any campus building, and correctly wear face masks outdoors when social distancing of at least six feet is not possible.
ABSTRACT
Deterministic culturing of single cells in 3D
Rohil Jain, Shirisha Chittiboyina, Chun-Li Chang, Sophie A. Lelièvre & Cagri A. Savran
DOI: 10.1038/s41598-020-67674-3
Models using 3D cell culture techniques are increasingly accepted as the most biofidelic in vitro representations of tissues for research. These models are generated using biomatrices and bulk populations of cells derived from tissues or cell lines. We present an alternate method to culture individually selected cells in relative isolation from the rest of the population under physiologically relevant matrix conditions. Matrix gel islands are spotted on a cell culture dish to act as support for receiving and culturing individual single cells; a glass capillary-based microfluidic setup is used to extract each desired single cell from a population and seed it on top of an island. Using examples of breast and colorectal cancers, we show that individual cells evolve into tumors or aspects of tumors displaying different characteristics of the initial cancer type and aggressiveness. By implementing a morphometry assay with luminal A breast cancer, we demonstrate the potential of the proposed approach to study phenotypic heterogeneity. Results reveal that intertumor heterogeneity increases with time in culture and that varying degrees of intratumor heterogeneity may originate from individually seeded cells. Moreover, we observe that a positive relationship exists between fast growing tumors and the size and heterogeneity of their nuclei.
Note to journalists: The paper is available online open-access at the journal’s website. An image and GIF of the 3D cell culture method are available via Google Drive. Journalists visiting campus should follow visitor health guidelines.

