July 27, 2020
‘Liquid biopsy’ tech contributes to successful clinical trial for detecting breast cancer recurrence
WEST LAFAYETTE, Ind. — Accurately diagnosing the spread of cancer often involves painful and invasive biopsy procedures. The use of a “liquid biopsy,” which involves a simple blood draw, has been shown in a five-year clinical trial to accurately detect and monitor certain kinds of breast cancer.
The study involves circulating tumor DNA (ctDNA) and circulating tumor cells (CTC), genetic and cellular material from tumors that find their way into the patient’s bloodstream. A paper on the clinical trial’s results is published in JAMA Oncology.
“These CTC cells are extremely rare,” said Cagri Savran, a professor of mechanical engineering at Purdue University. “In a blood sample of eight milliliters, there are billions of cells, but the cells we’re looking for, there may only be three or four.”
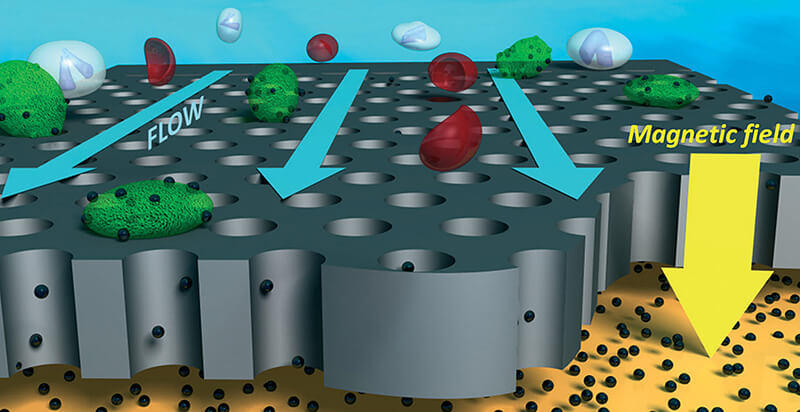
Savran and his team at the Birck Nanotechnology Center in Purdue’s Discovery Park developed a liquid biopsy device to isolate these cells. They mix blood samples with magnetic particles, functionalized with antibodies to recognize the cells they are targeting. Then they run the samples through a microfluidic device, which has a magnetic field that attracts and captures only the magnetized CTC cells.
“Once we’ve got them, we can do a number of things,” Savran said. “We can culture them, we can run tests on them and, most importantly for this trial, we can count them. The number of cells in a sample has a significant meaning.”
The device drew the attention of Milan Radovich, an associate professor at the IU Simon Comprehensive Cancer Center in Indianapolis and a 2004 Purdue graduate in biochemistry.
“I focus primarily on the use of genomics for precision medicine in oncology,” Radovich said. “We really focus on figuring out what makes cancers tick, by looking at their DNA blueprint.”
Radovich wanted to test the effectiveness of CTC detection as part of a larger clinical trial of 196 patients with triple-negative breast cancer.
 A “liquid biopsy” device developed by Purdue University engineers can accurately detect and monitor certain kinds of breast cancer. (Credit: Bin-Da Chan)
Download image
A “liquid biopsy” device developed by Purdue University engineers can accurately detect and monitor certain kinds of breast cancer. (Credit: Bin-Da Chan)
Download image
“This is a particularly aggressive form of breast cancer,” he said, “and recurrence of the disease tends to be high. So after their chemotherapy, we took blood samples from the patients to see if presence of the cells was an accurate marker of disease relapse.”
Because IU Simon Comprehensive Cancer Center doesn’t have CTC technology, Radovich reached out to Purdue to test the samples. “Our novel microfluidic platform makes CTC detection simple, rapid and scalable,” Savran said. “It takes longer to ship the samples than to complete the tests.”
From 2014 to 2018, Radovich sent hundreds of blood samples to Savran, who isolated the CTC cells and reported their numbers back to Radovich.
“Because this was a blinded study, neither of us could know the results until the trial was completed,” Radovich said. “It was a long five years to wait.”
In the final analysis, their theory was proven right.
“These circulating markers were more predictive of relapse than any other commonly used clinical marker on the planet,” Radovich said. “It’s more predictive than tumor size, grade, stage or lymph node status.”
“This is the largest clinical study ever published on the use of circulating markers and minimal residual disease,” Radovich said. “This really puts it on the map.”
A video describing this work is available on YouTube.
Both Radovich and Savran are now pursuing the future of non-invasive liquid biopsies. Savran patented the idea for his device through the Purdue Research Foundation Office of Technology Commercialization and created a company, Savran Technologies Inc., to make it commercially available.
“It’s very important to me and my team of engineers that the things we build are really useful,” Savran said. “This technology is being used on real patients and now has the power to make a real difference.”
Radovich plans to continue to use the technology in future clinical trials.
“Imagine you’re a patient with this triple-negative breast cancer,” Radovich said. “Even if chemotherapy and surgery were successful, life can be a nightmare. They’ve told us that every headache or back pain incites fear that their cancer is coming back. With this technology, we can offer a more definitive answer for both physicians and patients.”
Financial support for this study was provided by the Vera Bradley Foundation for Breast Cancer Research, Walther Cancer Foundation, Indiana University Grand Challenge Precision Health Initiative and Thomas Hurvis and McKinley Educational Foundation.
About Discovery Park
Discovery Park is a place where Purdue researchers move beyond traditional boundaries, collaborating across disciplines and with policymakers and business leaders to create solutions for a better world. Grand challenges of global health, global conflict and security, and those that lie at the nexus of sustainable energy, world food supply, water and the environment are the focus of researchers in Discovery Park. The translation of discovery to impact is integrated into the fabric of Discovery Park through entrepreneurship programs and partnerships.
About Purdue University
Purdue University is a top public research institution developing practical solutions to today’s toughest challenges. Ranked the No. 6 Most Innovative University in the United States by U.S. News & World Report, Purdue delivers world-changing research and out-of-this-world discovery. Committed to hands-on and online, real-world learning, Purdue offers a transformative education to all. Committed to affordability and accessibility, Purdue has frozen tuition and most fees at 2012-13 levels, enabling more students than ever to graduate debt-free. See how Purdue never stops in the persistent pursuit of the next giant leap at https://purdue.edu/.
Media contact: Kayla Wiles, wiles5@purdue.edu (working remotely, but will provide immediate response)
Writer: Jared Pike
Source: Cagri Savran, savran@purdue.edu
Journalists visiting campus: Journalists should follow Protect Purdue protocols and the following guidelines:
- Campus is open, but the number of people in spaces may be limited. We will be as accommodating as possible, but you may be asked to step out or report from another location.
- To enable access, particularly to campus buildings, we recommend you contact the Purdue News Service media contact listed on the release to let them know the nature of the visit and where you will be visiting. A News Service representative can facilitate safe access and may escort you on campus.
- Wear face masks inside any campus building. Wear face masks outdoors when social distancing of at least six feet is not possible.
ABSTRACT
Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer
DOI: 10.1001/jamaoncol.2020.2295
Importance A significant proportion of patients with early-stage triple-negative breast cancer (TNBC) are treated with neoadjuvant chemotherapy. Sequencing of circulating tumor DNA (ctDNA) after surgery, along with enumeration of circulating tumor cells (CTCs), may be used to detect minimal residual disease and assess which patients may experience disease recurrence.
Objective To determine whether the presence of ctDNA and CTCs after neoadjuvant chemotherapy in patients with early-stage TNBC is independently associated with recurrence and clinical outcomes.
Design, Setting, and Participants A preplanned secondary analysis was conducted from March 26, 2014, to December 18, 2018, using data from 196 female patients in BRE12-158, a phase 2 multicenter randomized clinical trial that randomized patients with early-stage TNBC who had residual disease after neoadjuvant chemotherapy to receive postneoadjuvant genomically directed therapy vs treatment of physician choice. Patients had blood samples collected for ctDNA and CTCs at time of treatment assignment; ctDNA analysis with survival was performed for 142 patients, and CTC analysis with survival was performed for 123 patients. Median clinical follow-up was 17.2 months (range, 0.3-58.3 months).
Interventions Circulating tumor DNA was sequenced using the FoundationACT or FoundationOneLiquid Assay, and CTCs were enumerated using an epithelial cell adhesion molecule–based, positive-selection microfluidic device.
Main Outcomes and Measures Primary outcomes were distant disease–free survival (DDFS), disease-free survival (DFS), and overall survival (OS).
Results Among 196 female patients (mean [SD] age, 49.6 [11.1] years), detection of ctDNA was significantly associated with inferior DDFS (median DDFS, 32.5 months vs not reached; hazard ratio [HR], 2.99; 95% CI, 1.38-6.48; P = .006). At 24 months, DDFS probability was 56% for ctDNA-positive patients compared with 81% for ctDNA-negative patients. Detection of ctDNA was similarly associated with inferior DFS (HR, 2.67; 95% CI, 1.28-5.57; P = .009) and inferior OS (HR, 4.16; 95% CI,1.66-10.42; P = .002). The combination of ctDNA and CTCs provided additional information for increased sensitivity and discriminatory capacity. Patients who were ctDNA positive and CTC positive had significantly inferior DDFS compared with those who were ctDNA negative and CTC negative (median DDFS, 32.5 months vs not reached; HR, 5.29; 95% CI, 1.50-18.62; P = .009). At 24 months, DDFS probability was 52% for patients who were ctDNA positive and CTC positive compared with 89% for those who were ctDNA negative and CTC negative. Similar trends were observed for DFS (HR, 3.15; 95% CI, 1.07-9.27; P = .04) and OS (HR, 8.60; 95% CI, 1.78-41.47; P = .007).
Conclusions and Relevance In this preplanned secondary analysis of a randomized clinical trial, detection of ctDNA and CTCs in patients with early-stage TNBC after neoadjuvant chemotherapy was independently associated with disease recurrence, which represents an important stratification factor for future postneoadjuvant trials.
Trial Registration ClinicalTrials.gov Identifier: NCT02101385
Note to journalists: A video of this work is available on YouTube. For a copy of the paper, please contact Kayla Wiles, Purdue News Service, at wiles5@purdue.edu. An illustration of the liquid biopsy technology is accessible via Google Drive. Journalists visiting campus should follow visitor health guidelines.

