Low oxygen boosts stem cell survival in muscular dystrophy therapy
August 21, 2012
 |
|
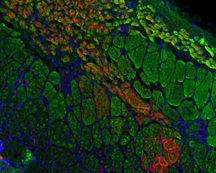
An image of a muscle
implanted shows pre-existing muscle fibers (green cells only), along with
fibers created by transplanted stem cells (green fiber with red membrane). Blue
areas represent cells' nuclei. (Purdue University image/Weiyi Liu and Shihuan
Kuang) |
WEST LAFAYETTE, Ind. - Controlling the amount of oxygen that stem cells are exposed to can significantly increase the effectiveness of a procedure meant to combat an often fatal form of muscular dystrophy, according to Purdue University research.
A genetic mutation in patients with Duchenne muscular dystrophy causes the constant breakdown of muscles and gradual depletion of stem cells that are responsible for repairing the damage and progressive muscle wasting. A healthy stem cell tends to duplicate in a regular pattern that creates one copy of itself that continues to function as a stem cell, and a differentiated cell, which performs a specific function. In a healthy person, a torn or damaged muscle would be repaired through this process.
Stem cell therapy - implanting healthy stem cells to combat tissue wasting - has shown promise against muscular dystrophy and other neurodegenerative diseases, but few of the implanted stem cells survive the procedure. Shihuan Kuang, a Purdue assistant professor of animal sciences, and Weiyi Liu, a postdoctoral research associate, showed that survival of implanted muscle stem cells could be increased by as much as fivefold in a mouse model if the cells are cultured under oxygen levels similar to those found in human muscles.
 |
|
Shihuan Kuang |
"Stem cells survive in a microenvironment in the body that has a low oxygen level," Kuang said. "But when we culture cells, there is a lot of oxygen around the petri dish. We wanted to see if less oxygen could mimic that microenvironment. When we did that, we saw that more stem cells survived the transplant."
 |
|
Weiyi Liu |
Liu thinks that's because the stem cells grown in higher oxygen levels acclimate to their surroundings. When they're injected into muscles with lower oxygen levels, they essentially suffocate.
"By contrast, in our study the cells become used to the host environment when they are conditioned under low oxygen levels prior to transplantation," Liu said.
In the mouse model, Kuang and Liu saw more stem cells survive the transplants, and those stem cells retained their ability to duplicate themselves.
"When we lower the oxygen level, we can also maintain the self-renewal process," Kuang said. "If these stem cells self-renew, they should never be used up and should continue to repair damaged muscle."
The findings, reported in the journal Development, shows promise for increasing the effectiveness of stem cell therapy for patients with Duchenne muscular dystrophy, which affects about one in 3,500 boys starting at about 3-5 years old. The disease, which confines almost all patients to wheelchairs by their 20s, is often fatal as muscles that control the abilities to breathe and eat deteriorate.
Xiaoqi Liu, a Purdue associate professor of biochemistry, and several graduate students contributed to the study.
Kuang's research will now focus on the signaling pathways within stem cells to understand how oxygen levels affect their functions and examining whether human muscle stem cells are similarly regulated by environmental oxygen. The National Institutes of Health, the Muscular Dystrophy Association and the U.S. Department of Agriculture funded the research.
Writer: Brian Wallheimer, 765-496-2050, bwallhei@purdue.edu
Sources: Shihuan Kuang, 765-494-8283, skuang@purdue.edu
Weiyi Liu, 765-494-485, liu98@purdue.edu
ABSTRACT
Hypoxia Promotes Satellite Cell Self-renewal and Enhances the Efficiency of Myoblast Transplantation
Weiyi Liu, Yefei Wen, Pengpeng Bi, Xinsheng Lai, X. Shawn Liu, Xiaoqi Liu and Shihuan Kuang
Microenvironmental oxygen (O2) regulates stem cell activity, and a hypoxic niche with low oxygen levels has been reported in multiple stem cell types. Satellite cells are muscle-resident stem cells that maintain the homeostasis and mediate the regeneration of skeletal muscles. We demonstrate here that hypoxic culture conditions favor the quiescence of satellite cell-derived primary myoblasts by upregulating Pax7, a key regulator of satellite cell self-renewal, and downregulating MyoD and myogenin. During myoblast division, hypoxia promotes asymmetric self-renewal divisions and inhibits asymmetric differentiation divisions without affecting the overall rate of proliferation. Mechanistic studies reveal that hypoxia activates the Notch signaling pathway, which subsequently represses the expression of miR-1 and miR-206 through canonical Hes/Hey proteins, leading to increased levels of Pax7. More importantly, hypoxia conditioning enhances the efficiency of myoblast transplantation and the self-renewal of implanted cells. Given the robust effects of hypoxia on maintaining the quiescence and promoting the self-renewal of cultured myoblasts, we predict that oxygen levels in the satellite cell niche play a central role in precisely balancing quiescence versus activation, and self-renewal versus differentiation, in muscle stem cells in vivo.
Ag Communications: (765) 494-2722;
Keith Robinson, robins89@purdue.edu
Agriculture News Page

