March 27, 2018
New glass-like polymer could conduct electricity for transparent electronics
WEST LAFAYETTE, Ind. — Purdue researchers have created a transparent polymer film that also conducts electricity, introducing an inexpensive organic material for applications such as the screens of electronic devices.
While some polymers can already conduct electricity with the help of a process called chemical doping, none have yet been made that conduct just as well in a transparent form. This combination could find use in TV, phone and computer screens that currently use a relatively expensive inorganic material, indium tin oxide, to serve as a transparent conductor. The researchers detail their discovery in a paper published on March 23 in Science.
“The precursors of this new polymer are made on the ton scale,” said Bryan Boudouris, the Robert and Sally Weist Associate Professor of Chemical Engineering. “Having a conducting transparent material from carbon-based materials would be significant.”
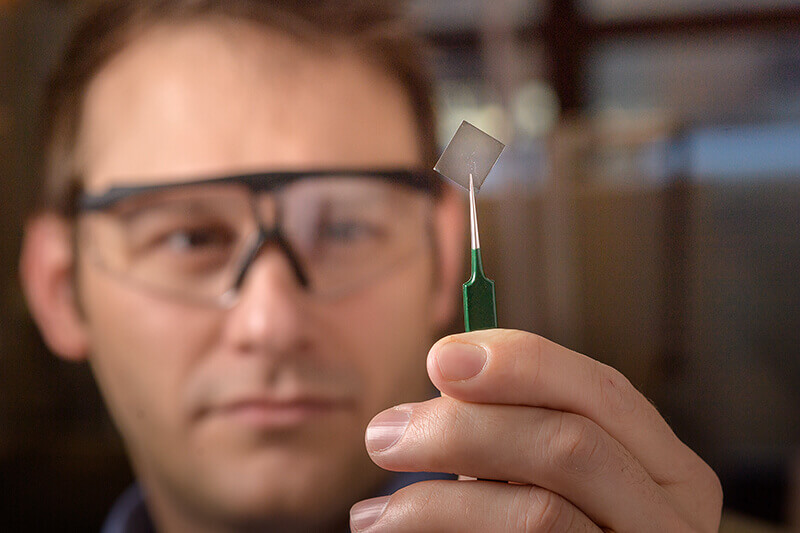 Purdue researcher Brett Savoie holds a transparent polymer film that conducts electricity inexpensively and easily. (Purdue University image/John Underwood)
Download image
Purdue researcher Brett Savoie holds a transparent polymer film that conducts electricity inexpensively and easily. (Purdue University image/John Underwood)
Download image
The polymer film, which has the look and feel of glass, can be more cheaply produced on a large scale than indium tin oxide because it originates from earth-abundant materials. Its cost effectiveness also has advantages over polymers already used for electronics that rely on expensive chemistry and chemical doping to achieve high conductivity. Existing conducting polymers also typically absorb light due to their conjugated, or delocalized, electron network structure.
“It’s tough to engineer something that is conjugated to be transparent,” said Brett Savoie, an assistant professor of Chemical Engineering. “So, we started with a completely new non-conjugated polymer and modified it to conduct.”
Modifications involved adding radical groups that are already transparent but typically wouldn’t conduct electricity within a polymer. “Our idea was, could we use polymers easier to make on a large scale but also get them to be electronically active?” Boudouris said.
 This transparent electronic film is an organic material that could be used for the screens of devices. (Purdue University image/Yongho Joo)
Download image
This transparent electronic film is an organic material that could be used for the screens of devices. (Purdue University image/Yongho Joo)
Download image
Savoie simulated how the radical groups electronically communicate with each other across the polymeric material, a property known as electrical percolation, to figure out how to make the polymer a good conductor. The simulations showed that it was critical to incorporate more radicals than previous studies had been able to achieve, and also make the polymer soft enough that the radical groups could associate within the material. Boudouris accomplished this by synthesizing a material that had both a high density of radical sites and could flow at low temperatures, resulting in a transparent film processed near room temperature.
This polymer is not only transparent, but could conduct electricity competitively with commercially available conjugated polymers. “The simulations gave us the design principles that will allow us to make even better conductors in the future,” Boudouris said. A YouTube video explaining this concept is available at https://www.youtube.com/watch?v=b-TJJDvdBvE&feature=youtu.be.
 An artistic rendering shows how a high density of transparent radical groups (light regions) enable a polymer to conduct electricity despite non-conducting areas (dark regions). (Purdue University image/Brett Savoie)
Download image
An artistic rendering shows how a high density of transparent radical groups (light regions) enable a polymer to conduct electricity despite non-conducting areas (dark regions). (Purdue University image/Brett Savoie)
Download image
The new polymer also provides insights for future research to be conducted by Boudouris, Savoie and other faculty at the Purdue-based Materials Innovation for Bioelectronics from Intrinsically-stretchable Organics (Mi-Bio) center, which aims to produce tailor-made polymers for biomedical applications.
“Conducting polymers are different from other conductors in that they can reversibly exchange ions and analytes with liquid media—kind of like a sponge—and this creates all kinds of interesting sensing and device applications.” Savoie said.
The research, performed by both Purdue’s Davidson School of Chemical Engineering and Department of Chemistry, is funded by the National Science Foundation and the Air Force Office of Scientific Research.
Writer: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Sources: Bryan Boudouris, 765-496-6056, boudouris@purdue.edu
Brett Savoie, 765-494-4235, bsavoie@purdue.edu
Note to Journalists: For a full-text copy of the paper, please contact Kayla Wiles, Purdue News Service at wiles5@purdue.edu. A YouTube video is available at https://www.youtube.com/watch?v=b-TJJDvdBvE&feature=youtu.be and other multimedia can be found in a Google Drive folder at https://drive.google.com/drive/folders/1PFYSBr04hHvXtqblY_tISCutuBXfXlkE. The materials were prepared by Erin Easterling, digital producer for the Purdue College of Engineering, 765-496-3388, easterling@purdue.edu.
ABSTRACT
A nonconjugated radical polymer glass with high electrical conductivity
Yongho Joo,1* Varad Agarkar,2* Seung Hyun Sung,1
Brett M. Savoie,1 Bryan W. Boudouris1,2
1Charles D. Davidson School of Chemical Engineering, Purdue University
2Department of Chemistry, Purdue University
doi: 10.1126/science.aao7287
Solid-state conducting polymers usually have highly conjugated macromolecular backbones and require intentional doping in order to achieve high electrical conductivities. Conversely, single-component, charge-neutral macromolecules could be synthetically simpler and have improved processibility and ambient stability. We show that poly(4-glycidyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl), a nonconjugated radical polymer with a subambient glass transition temperature, underwent rapid solid-state charge transfer reactions and had an electrical conductivity of up to 28 siemens per meter over channel lengths up to 0.6 micrometers. The charge transport through the radical polymer film was enabled with thermal annealing at 80°C, which allowed for the formation of a percolating network of open-shell sites in electronic communication with one another. The electrical conductivity was not enhanced by intentional doping, and thin films of this material showed high optical transparency.

