Thermodynamics
 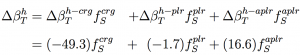 |
Our research group has exploited computational methods to probe the molecular origins that stabilize protein conformations and protein-ligand complexes. Recent work in the group couples NMR with simulation methods to rationalize entropy/enthalpy compensation measured for ligands binding SH2 domains. In addition, an assimilation of results for a set of globular proteins obtained from molecular dynamics simulation with thermodynamic experiment lead to a new proposal that protein intrinsic compressibility varies depending on the distribution of charged groups between the protein surface and the protein core: the adhesive-cohesive model. Further, this work realized a correlation of protein intrinsic compressibility with enthalpic stability, which was the basis our proposal that the evolution of enthalpic stability in globular proteins is based on burying charged groups in the protein interior, a notion which is counter-intuitive given the strong solvation energy associated with charged groups. How do buried charges stabilize protein structure??
|

