Pore space in soilless substrates
Do you know that you are mostly paying for ‘pore space’ when buying a bag of soilless substrate? This is because, unlike mineral soils, nearly three-fourth volume of soilless substrates is pore space. I like to refer pore space as the ‘functional’ component of a substrate. By definition, pore space is the volume available for air and water in a substrate, i.e. substrate volume minus solids. In other words, a substrate with 75% pore space contains 25% of solids. Wide pores are good for air exchange while narrow pores hold water. Ideally, air space should be 20-25% of pore space for proper root growth. Therefore, it is important to understand pore space and maintain a balance between air and water space in a substrate.
How do you measure pore space?
Pore space is expressed in terms of ‘porosity’. Use the following steps to measure porosity, air space and water space in your substrate:
(i). Find out the volume of the pot (usually obtained from the manufacturer).
(ii). Add a water-tight liner to the outer bottom of the pot. Tape the liner to the pot to prevent any drainage through the holes.
(iii). Fill the substrate to the top of the pot. Make sure to compact the substrate normally as you do during production. Substrate volume is now equal to the volume of pot.
(iv). Measure the volume of water needed to completely saturate the substrate (until you start to see a shiny layer of water over the substrate). The volume of water needed to saturate the substrate is equivalent to the ‘total pore space.
(v). Place the pot in a water-tight container and make holes at the bottom by puncturing the liner. This will let water to drain out of the pot. Leave 10-15 min for water to completely drain from the pot and then measure the drained volume. This volume is equivalent to the ‘air space volume’. From this, porosity (%), air space (%) and water space (%) can be calculated as follows:
Porosity (%) = 100 * (Total pore volume/Substrate volume)
Air space (%) = 100 * (Air space volume/Total pore volume)
Water space (%) = 100 – Air space (%)
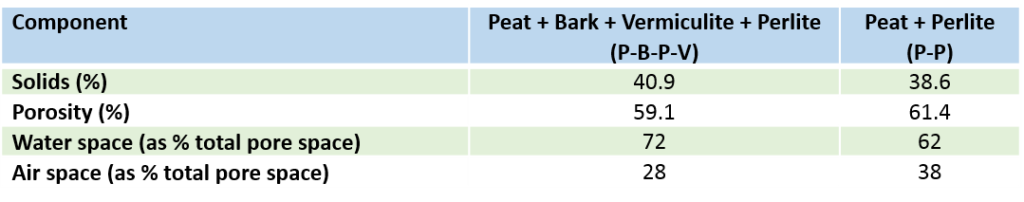
Following table provides information on fractional volume of pore space, air space and water space in two soilless substrates (Nemali et al, 2005):
Pore space is dynamic
Pore space is not a constant characteristic of the substrate. Pore space can change with (i) expansion and contraction of the organic components (ex: peat) during watering and drying, (ii) decomposition of organic matter which results in a change in the particle size of the substrate (changes pore size), and (iii) compaction of substrate due to overhead irrigation or root growth which results in interlocking of substrate particles or increases bulk density (reduces pore space). Therefore, measurements of pore space based on a fresh substrate should be cautiously used to describe the substrate. The approach described above can used to measure the dynamic changes in pore space during crop growth as well.
Maximum water-holding or container capacity
Water space (%) calculated above is also called ‘maximum water-holding capacity’ or ‘container capacity’ of the substrate. It is defined as the percentage of total pore space that is occupied by water after drainage is complete from the substrate (after air occupies pore space). Water holding capacity is not same as the volume of water required for saturation of the substrate. The main difference is that, all pores are filled with water at saturation while air replaces water in larger pores at container capacity. Water is held inside the narrow pores against gravity by capillary forces. Water inside the large pores drains because force of gravity is greater than capillary force in these pores. Water holding capacity is affected by total pore space, therefore it is considered as a dynamic property of a substrate. It can change due to substrate type, compaction and root growth. Container height is another characteristic that influences water-holding capacity. For a given diameter, water-holding capacity decreases with increasing container height due increased effect of gravity on water column. For a given height, water-holding capacity increases with diameter of the container. In spite of drainage holes, some volume of soil at the bottom of the container never drains. This is due to relatively less influence of gravity on substrate water in this zone. This zone is called ‘perched water zone’. Air space is near zero in this zone. Perched zone becomes a larger portion of total soil volume as container height decreases. This is one reason why it is challenging to grow uniform quality seedlings in small plug cells.
Figure 1: Effect of container height on air and water space in soilless substrates

Pore sizes
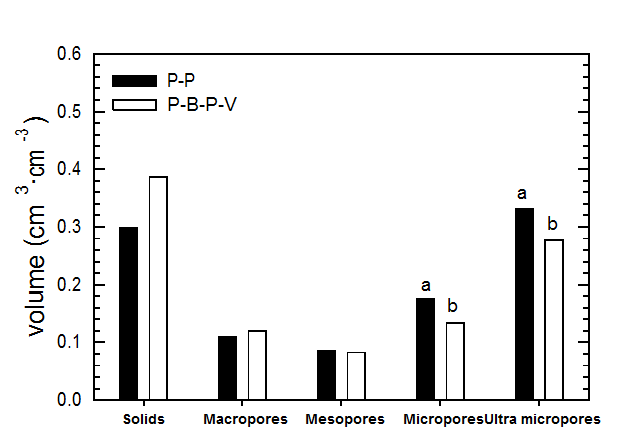
Substrate pores are designated as inter-aggregate or macropores for water infiltration/drainage and intra-aggregate or meso/micropores for water retention. Most of the water retained in macropores is usually lost in drainage, which lowers the substrate water status from saturation to water-holding capacity. The easily available water for plants in soilless substrates is usually retained in the meso- and micropores. Pores sizes are categorized into different classes based on the Soil Science Society of America pore size classification system (Kay and Angers, 2001). Based on this, macropores are considered to have a radius greater than 0.0075 cm, mesopores to have a radius in the range of 0.003 to 0.0075 cm, micropores to have a radius in the range of 0.003 to 0.0003 cm, and ultra-micropres to have a radius less than 0.0003 cm. Water held by ultra-micropores is usually unavailable to plants. Following figure (Nemali et al., 2005) provides information on fractional volume of different pore sizes in peat + Perlite (P-P) and Peat + Bark + Perlite + Vermiculite (P-B-P-V) substrates. Note a significant increase in micro and ultra-micropores in P-P than P-B-P-V substrate. This is likely due to more peat content in P-P than P-B-P-V substrate. Peat fibers contain narrow pores and increase micropore space.
Figure 2. Different pore fractions in peat + perlite (P-P) and peat+ bark + perlite + vermiculite (P-B-P-V) substrates. Different letters indicate statistical significance.
Available and unavailable water
Not all water in the pores is available to plants. Water in meso- and micropores is generally available while water in ultra-micropores is held so tightly that it is difficult to be extracted by the roots. Water molecules are held together by both cohesive (water-water) and adhesive (water-solids) forces inside pores. Root hairs should exert sufficient pressure to break these forces and extract water from the pores. For this, a gradient in pressure between the soil solution and roots should develop for water to move along the pressure gradient, i.e., from high water (substrate solution) to low water (roots) pressure. Roots maintain a negative pressure relative to the substrate solution as water moves upwards from roots and lost in transpiration. When necessary roots can maintain more negative pressure by synthesizing complex carbohydrates produced in photosynthesis. However, this process comes at a cost to plants. Cohesive forces are relatively easy to break than adhesive forces. Generally, adhesive forces dominate inside the ultra-micropores due to narrow pore size and thin water columns. Sometimes it can be difficult for roots to develop enough negative pressure to break the adhesive forces and extract water from ultra-micropores. This is the reason why it is hard to extract water from ultra-micropores. Therefore, presence of large number of ultra-micropores can increase the amount of unavailable water in the substrate. Compacting a substrate not only reduces air space but also proportion of unavailable water in the substrate by increasing ultra-micropores. Bigelow et al. 2000 reported that pure peat moss has large volume of unavailable water due to large fraction of ultra-micropores (inside peat fibers). This is why peat based substrates should be compacted lightly to avoid further increase in ultra-micropores.
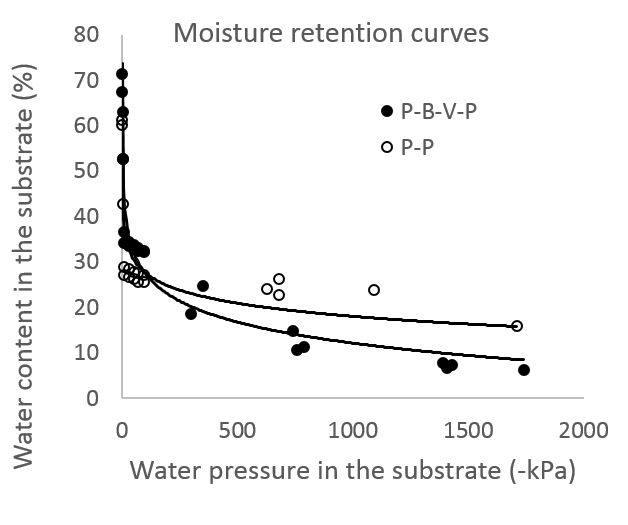
Available water can be estimated as the difference in water volume at container capacity and wilting point. Generally ‘substrate moisture retention curves’ are used to determine characteristics like plant available water. When the pressure with which water held inside the pores falls below -1500 kPa (kilo Pascal), then the water is assumed to be unavailable to plants. At this pressure, plants start to show wilting signs. In the figure below, the moisture retention curves for peat-perlite (P-P) and peat-bark-perlite-vermiculite (P-B-P-V) substrates are shown. These curves indicate the water content in the substrate at different pressures inside the pores. Note that most of the water in the two soilless substrates is held at pressure below -500 kPa. This means that most of the water in the pores can be easily extracted by the roots; however the fact that most of the water is within a short range (0 to -500 kPa) means that roots can rapidly shift from no stress situation to moderate water stress as water dries down in the substrate. Also important to note from the graph is that nearly 20 and 10% of water in the P-P and P-B-P-V substrates, respectively is held at pressures below -1500 kPa. These water volumes are likely unavailable to roots.
Figure 3. Substrate moisture retention curves for peat + perlite (P-P) and peat+ bark + perlite + vermiculite (P-B-P-V) substrates.
Substrate moisture retention curves require complicated equipment for measurement. However, a simpler method to measure volume of available water in soilless substrates is described below:
(i). Select 4-6 containers with plants and measure container weight at maximum water holding capacity (W1, grams). Make sure to irrigate substrate uniformly and slowly from the top till it is close to saturation. Allow sufficient time for drainage prior to weighing.
(ii). Stop watering plants in the selected containers after measurement and allow the substrate to dry down for a few days till you observe mild wilting signs on leaves.
(iii). Measure the weight of containers again at mild wilting stage (W2, grams).
(iv). Calculate average of W1 and W2 weights.
(iv). The difference between average W1 and W2 is the available water in the substrate. Convert weight to volume by using 1000 g of water = 1 L of water.
Hydraulic conductivity
The ease with which water can move through the pores is described as hydraulic conductivity. In peat- based substrates, a sharp decline in hydraulic conductivity or increase in the resistance to water flux has been observed within a narrow range of water status in the substrate. A 100-fold decrease in hydraulic conductivity was observed when water content decreased from 60 to 20% in peat media (da Silva et al.1993). What this means is that, in spite of reasonable volume of water in the substrate, increased resistance to water flux due to drop in hydraulic conductivity will reduce water availability to plants. Therefore, hydraulic conductivity should be considered along with water content of the substrate for irrigation scheduling. Drying peat-based substrates between irrigations and adding large volume of water during each irrigation will only result in leaching of irrigation water from containers with a little volume retained in the substrate. This is due to large decrease in hydraulic conductivity of peat when it is dry. If the substrate is dry, water in small volumes with sufficient time between irrigations to hydrate the medium and increase hydraulic conductivity.
References
- Bigelow, C.A., D. Bowman and K. Cassel. 2000. Sand-based rootzone modification with inorganic soil amendments and sphagnum peat moss. USGA Green Section Record 38 (4), 7-13.
- da Silva, F.F., R. Wallach and Y. Chen. 1993. Hydraulic properties of sphagnum peat moss and tuff (scoria) and their potential effects on water availability. Plant and Soil. 154: 119-126.
- Hillel, D. 1982. Introduction to soil physics. Academic Press, Inc. San Diego.
- Kay, B.D., and D.A. Angers. 2001. Soil structure. M.E. Summner (ed.). Handbook of soil science. CRC Press. Boca Raton, FL.
- Nemali, K.S., D.E. Radcliffe, and M.W. van Iersel. 2005. Moisture retention curves and pore size distribution in soilless substrates. Chapter 4. Ph.D. Dissertation, University of Georgia.