Protein determines life or death fate of stressed cells
April 1, 2015
 |
|
Seema Mattoo, an assistant professor of biological sciences, stands next to graduate student Anwesha Sanyal in Mattoo's Purdue laboratory. Mattoo and Sanyal led a research team that discovered a new protein involved in the process that determines the fate of cells under stress. (Purdue University photo/Charles Jischke) |
WEST LAFAYETTE, Ind. - Researchers discovered a new protein involved in the process that determines the fate of cells under stress and whether they fight to survive or sacrifice themselves for the greater good.
A protein named HYPE orchestrates a response to misfolded proteins within the cell, mistakes which increase when a cell is under stress from disease or injury, said Seema Mattoo, an assistant professor of biological sciences at Purdue University who led the research.
Correct protein folding dictates the structure of a protein, which is as important to its ability to function as the molecules that make it up, she said. It also is critical in maintaining a stable environment within the endoplasmic reticulum, a structure within the cell responsible for protein synthesis.
The cellular pathway that maintains this process in response to stress is called the unfolded protein response, she said.
Like a person folding a pile of clothes fresh from the dryer, proteins within the endoplasmic reticulum usually handle the chore of protein folding neatly and methodically. Sometimes mistakes are made and items need to be refolded. Usually, the amount of refolding needed is manageable and efficiently finished, but stress on the cell leads to an increase in misfolded proteins and, like the laundry, misfolded proteins can pile up. Unlike the laundry, however, how well a cell keeps up with the task of refolding proteins is a matter of life and death.
 |
|
Seema Mattoo, an assistant professor of biological sciences, shows a slide of the HYPE protein in human epithelial cells on an inverted fluorescence microscope designed for cell imaging. (Purdue University photo/Charles Jischke) |
"If a cell is stressed and misfolded proteins begin to occur more frequently, the cell tries to pick up the pace and refold the proteins in an effort to survive," Mattoo said. "If the stress is sustained, more misfolds occur and eventually the cell can't keep up with the necessary refolding. At this point, a switch is flipped that activates cell death. This isn't always a bad thing, as it is a mechanism that can help prevent the spread of infection and offer benefits to the surrounding cells."
How well a cell copes with refolding proteins depends on a protein named BiP, which binds to misfolded proteins and tries to refold them, she said.
"It was thought that BiP acted as the sole sentinel of quality control for the protein assembly line in the endoplasmic reticulum," Mattoo said. "Now we know HYPE can manipulate the BiP protein and can alter the stress response. It influences whether a path of survival or path of self-induced death is taken."
The BiP protein has been identified as a potential therapeutic target for cancer and heart disease and HYPE may offer another way to achieve the same therapeutic effects, Mattoo said.
"Diseased cells are under stress and HYPE could be a new target for therapeutic drugs," Mattoo said. "An increase in HYPE has been seen in some cancers. Perhaps the cancer cells need it to deal with the stress, and blocking the protein would slow progression of the disease. We don't know its potential at this point, but identifying its role opens the door to discovering how it might be used to prevent and treat disease."
A paper detailing the work was published in the current issue of The Journal of Biological Chemistry and is available online. In addition to Mattoo, co-authors include Purdue graduate students Anwesha Sanyal and Andy J. Chen; Purdue staff member Erica A. Zbornik; Ernesto S. Nakayasu, an infectious disease systems biologist at Purdue Bindley Bioscience Center; Cheri S. Lazar and Carolyn A. Worby from the Department of Pharmacology at the University of California-San Diego; and Antonius Koller from the Department of Pathology at Stony Brook University.
 |
|
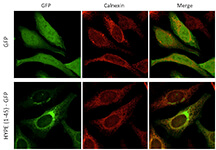
Localization of the HYPE protein in the endoplasmic reticulum of human epithelial cells. HYPE is illuminated with GFP, a green marker, and the endoplasmic reticulum is illuminated with calnexin, a red marker. (Purdue University image/courtesy of Seema Mattoo) |
The team found that HYPE modifies BiP by adenylylation, a process that involves the attachment of an adenosine monophosphate obtained from the cell's ATP stocks. This enhances BiP's ATPase activity, which is required for refolding misfolded proteins while coping with stress. The team also determined that a suppression of HYPE prevents an optimal response to misfolded proteins.
"We found that HYPE modifies BiP and that this ultimately alters the stress response pathway taken, but exactly how it accomplishes this is unknown," Mattoo said. "HYPE might increase the ability of BiP to bind to misfolded proteins or its ability to refold the proteins, or it may increase the ability of BiP to dissassociate from other proteins that activate different branches of the unfolded protein response pathway. There is much more to learn."
HYPE is a member of a family of proteins called Fic proteins, found in many living organisms. HYPE is the only known human Fic protein and its function in human cells was unkown, she said.
"Now we know that HYPE resides inside the endoplasmic reticulum and that it acts as a regulator of protein folding," Mattoo said. "This work provided the first demonstration of the physiological significance of the human Fic protein, HYPE, and its role in a cell signaling process."
The team next will study whether different stress signals elicit different responses and will try to identify the signal that activates HYPE, she said.
"There are hundreds of proteins for which we have no idea of what they do," Mattoo said. "Studying proteins is a little like playing with Russian dolls. When you investigate the role of one, it often leads to another protein and something significant about its role. It goes on and on, and, as a researcher, I follow where it takes me."
The findings of Mattoo and her team have been well received. The paper is among the Journal of Biological Chemistry's top five most downloaded articles.
The American Cancer Society and Purdue Center for Cancer Research, Indiana Clinical and Translational Science Institute, Showalter Research Trust, Purdue Research Foundation and Purdue Department of Biological Sciences funded the research.
Writer: Elizabeth K. Gardner, 765-494-2081, ekgardner@purdue.edu
Source: Seema Mattoo, 765-494-0876, smattoo@purdue.edu
ABSTRACT
A Novel Link Between Fic (Filamentation induced by cAMP)-mediated Adenylylation/AMPylation and the Unfolded Protein Response
Anwesha Sanyal, Andy J. Chen, Ernesto S. Nakayasu, Cheri S. Lazar, Erica A. Zbornik, Carolyn A. Worby, Antonius Koller and Seema Mattoo
Maintenance of ER (endoplasmic reticulum) homeostasis is a critical aspect of determining cell fate and requires a properly functioning unfolded protein response (UPR). We have discovered a hitherto unkown role of a post-translational modification termed adenylylation/AMPylation in regulating signal transduction events during UPR induction. A family of enzymes, defined by the presence of a Fic (filamentation induced by cAMP) domain, catalyzes this adenylylation reaction. The human genome encodes a single Fic protein, called HYPE (Huntingtin yeast interacting protein E), with adenylyltransferase activity but unkown physiological targets. Here, we demonstrate that HYPE localizes to the lumen of the endopasmic reticulum via its hydrophobic N-terminus, and adenylylates the ER molecular chaperone, BiP, at Ser365 and Thr366. BiP functions as a sentinel for protein misfolding and maintains ER homeostasis. We find that adenylylation enhances BiP’s ATPase activity, which is required for refolding misfolded proteins while coping with ER stress. Accordingly, HYPE expression levels increase upon stress. Further, siRNA-mediated knockdown of HYPE prevents the induction of an unfolded protein response. We, thus, identify HYPE as a new UPR regulator and provide the first functional data for Fic-mediated adenylylation in mammalian signaling.

