New visualization reveals virus particles have more individuality than thought
May 28, 2013
 |
|
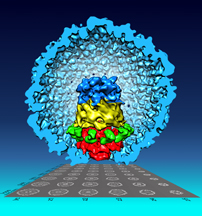
The bacteriophage T7 procapsid structure and the DNA packaging apparatus at the portal vertex was reconstructed using single particle cryo-EM and the new Focused Asymmetric Reconstruction (FAR) image processing technique developed by Wen Jiang, a Purdue University associate professor of biological sciences. Using the technique, a team led by Jiang revealed that the layers at the portal vertex are assembled in tandem with distinct symmetries and are also tilted slightly off-axis to facilitate DNA packaging without tangling. (Purdue University image/Fei Guo) |
WEST LAFAYETTE, Ind. - Virus particles of the same type had been thought to have identical structures, like a mass-produced toy, but a new visualization technique developed by a Purdue University researcher revealed otherwise.
Wen Jiang, an associate professor of biological sciences, found that an important viral substructure consisted of a collection of components that could be assembled in different ways, creating differences from particle to particle.
"It was assumed that each individual virus particle was an exact copy of the others, but nature is more complicated than that," Jiang said. "There are actually slight differences that result in a number of potential structural combinations for this part of the virus. By looking at only one very small part of this substructure at a time, we were able to obtain clear images and show various different combinations an individual particle could possess. Better visualization allows us to better understand the structure of viruses, which could lead to new ways to treat infections and improve human health."
Visualization of a virus particle's structure relies on a reconstruction created from views of many different particles. Scientists captured images of particles in a thin layer of solution, each frozen in a different orientation, like toys from an assembly line spread out haphazardly on a blanket. Some are facing up and others down, while some are on their side and others are on end. By combining all of the views, one could get a good idea of what the whole toy looks like. In this way, the structure of a single virus is created from the averages of the group.
However, this only works if the toys or particles are identical. When there are variations and different combinations of parts possible, a reconstruction from views of different individuals tends to be distorted and creates an inaccurate representation.
Because of the distortions that occur when the group cannot be averaged together, current visualization methods would often result in portions of the structure appearing blurred or smeared, Jiang said.
In his pursuit of improved imaging, Jiang studied the bacteriophage T7, a virus that infects bacteria. He focused on a small substructure, called the portal vertex, to see if it was identical across individual particles. The portal vertex is similar to a stack of five rings, where each ring is made of several copies of a single protein molecule that is different for each ring, he said.
He found that when he focused only on one pair of neighboring rings at a time, the computer analysis and averaging worked, and a clear image was obtained. When he tried to include three or more rings, the images were smeared.
This is because the five different rings had four different symmetries. Each ring, in essence, had a different shape. One ring had five points, two had 12, one had eight and one had four. Each ring could be rotated slightly, causing it to line up differently with the ring above and below it, he said.
"If you only consider a pair of two neighboring rings, the different alignments are structurally equivalent and can be averaged," Jiang said. "Whether point A of one ring aligns with point A, B or C on its neighbor, the structural symmetry is the same. For that one small part of the structure, the particles are identical. If you add in a third ring though, the rotation of a ring changes the symmetry of the overall structure and different particles are no longer identical."
He named the new imaging method "focused asymmetric reconstruction," or FAR, because it focuses in on a single neighboring pair of components. The focus can be moved around to resolve each of the neighbor pairs separately, and Jiang and his team were able to obtain clear images of the different structural options for each layer of the stack. The different options could then be put together to show different possible combinations for the larger structure, he said.
A paper detailing the imaging technique and findings of the National Institutes of Health-funded research was published in the journal of the Proceedings of the National Academy of Sciences. In addition to Jiang, members of the research team include Fei Guo, Zheng Liu, Frank Vago, Yue Ren and Weimin Wu of Purdue's Department of Biological Sciences and Elena Wright and Philip Serwer of the Department of Biochemistry at the University of Texas Health Science Center in San Antonio.
The portal vertex is involved in DNA packaging, and through the images obtained, the team discovered that the portal vertex is slightly tilted off of the axis, like a spinning top, regardless of the different combinations of the rings, Jiang said.
"The tilt of this structure could help the virus process the DNA and carefully coil it as it is fed into the empty procapsid shell, or head, of the virus," he said. "It appears there may also be the ability for some motion, which, coupled with the tilt, could create a spiral in the strand of DNA as it passes through. This would coil the DNA and prevent it from tangling."
The new visualization technique makes possible the analysis of layered structures with interfaces that vary in relation to each other and layers that move. The team plans to use the new visualization method to determine the movements of the motor that drives DNA packaging, Serwer said.
"All living organisms have either RNA or DNA, which would be inactivated by either tangling or knotting," Serwer said. "The tangling problem becomes more graphic when you consider that in each of our cells approximately five centimeters of DNA, on average, is tightly packed into each chromosome. As in the past, scientists use bacteriophages to simplify the understanding of phenomena like the avoidance of DNA tangling. Analysis of these phenomena involves a combination of basic physics and primitive evolution. We never know exactly when understanding of phenomena like this will have practical application."
However, bacteriophages themselves have been and can now be used to treat bacterial infections, he said.
"Bacteriophages were successfully used to treat infections before the discovery of antibiotics," he said. "When bacteriophages were well chosen and well used, they did a remarkable job of destroying the bacteria without harming the patient. Currently, the use of bacteriophages has the potential to be the most rapid response in the treating of antibiotic-resistant bacteria. These bacteria sometimes cause devastating infections. The more we know about bacteriophages, the greater the chance is that we will use them optimally."
The images were taken in the Purdue Biological Electron Microscopy Facility on the Titan Krios instrument. Purdue's Rosen Center for Advanced Computing provided the computational resources for the reconstructions.
Writer: Elizabeth Gardner, 765-494-2081, ekgardner@purdue.edu
Sources: Wen Jiang, 765-496-8436, jiang12@purdue.edu
Philip Serwer, 210-567-3765, serwer@uthscsa.edu
ABSTRACT
Visualization of Uncorrelated, Tandem Symmetry Mismatches in the Internal Genome Packaging Apparatus of Bacteriophage T7
Fei Guo, Zheng Liu, Frank Vago, Yue Ren, Weimin Wu, Elena T. Wright, Philip Serwer, and Wen Jiang
Motor driven packaging of a dsDNA genome into a pre-formed protein capsid through a unique portal vertex is essential in the life cycle of a large number of dsDNA viruses. We have used single-particle electron cryo-microscopy to study the multi-layer structure of the portal vertex of the bacteriophage T7 procapsid, the recipient of T7 DNA packaging. Focused asymmetric reconstruction (FAR) method was developed and applied to selectively resolve neighboring pairs of symmetry-mismatched layers of the portal vertex. However, structural features in all layers of the multi-layer portal vertex could not be resolved simultaneously. Our results imply that layers with mismatched symmetries can join together in several different relative orientations, and that orientations at different interfaces assort independently to produce structural isomers, a process that we call combinatorial assembly isomerism. This isomerism explains rotational smearing in previously reported asymmetric reconstructions of the portal vertex of T7 and other bacteriophages. Combinatorial assembly isomerism may represent a new regime of structural biology in which globally varying structures assemble from a common set of components. Our reconstructions collectively validate previously proposed symmetries, compositions and sequential order of T7 portal vertex layers, resolving in tandem the 5-fold gp 10 shell, 12-fold gp8 portal ring, and an internal core stack consisting of 12-fold gp14 adaptor ring, 8-fold bowl-shaped gp15, and 4-fold gp16 tip. We also found a small tilt of the core stack relative to the icosahedral 5-fold axis, and propose that this tilt assists DNA spooling without tangling during packaging.

