

January 22, 2008
New technique quickly detects cancer indicator
WEST LAFAYETTE, Ind. - |
By combining two distinct techniques, the technology can examine large numbers of cells individually, a feat not previously possible, said Chang Lu, a Purdue University assistant professor of agricultural and biological engineering.
"We really have bridged the gap between different
 |
In a study published this month in Analytical Chemistry, Lu demonstrated that the technique can detect a handful of protein movements, or translocations, within entire populations of cells.
These movements are important to detect because they are involved in many disease processes, such as oncogenesis, wherein a normal cell becomes malignant, said Robert Geahlen, a study co-author and researcher in the Department of Medicinal Chemistry and Molecular Pharmacology.
"Protein translocations are involved in the activation of tumor cells," he said. "Detecting these movements could help diagnose the type and stage of cancer in the future."
Lu's method uses two existing technologies: electroporation - used to determine protein location - and flow cytometry, a technique capable of rapidly examining individual cells but blind to intracellular protein locations on its own.
The Purdue technique, called "electroporative flow cytometry," harnesses the discerning power of the first method with the speed of the second, Geahlen said.
The method involves cells being sent through tiny channels within a microchip and undergoing electroporation, wherein electrical pulses open pores in cell membranes and protein is released from inside. Then, sensors measure protein concentrations. Since a protein's subcellular location can directly influence the amount of protein leaving the cell, as Lu and Geahlen have shown, this method is capable of indirectly determining location, Geahlen said.
If proteins are in their original position, floating freely in the cell's interior, or cytoplasm, a large percentage of them will flow out of the cell upon electroporation, Lu said. If translocation has occurred, in which proteins migrate from the cytoplasm to tightly bind to the interior of the cell membrane, few will be able to leave.
Previous technologies detect either protein movement in a few individual cells via slow imaging techniques or take average measurements from larger groups of cells, data usually irrelevant to protein location in individual cells, Lu said.
"When looking at a few cells, you see the trees but not the forest," he said. "When you take average measurements from large groups, you see the forest but not the trees. Our method allows us to know something about each tree in the forest."
The technology has the potential to be scaled up significantly, Lu said. In the study, 100-200 cells were processed per second, but that rate could potentially increase to speeds typical of flow cytometry, which goes through 10,000 cells per second. Speed increases can be achieved by optimizing the technology he said.
The study examines the movement of a certain type of protein called kinases. Kinases and their translocations are important for activating and deactivating cells and sending signals to one another, Geahlen said.
"There are many important kinases, enzymes and other proteins that become activated by translocation to the plasma membrane, and we've shown that we can detect one type of translocation," he said. "It's a first step."
Lu has filed a provisional patent for the technique and said that he could see it being used in a clinical setting in five to 10 years.
Study co-authors include graduate students Jun Wang, Leela Paris, Hsiang-Yu Wang, and postdoctoral researcher Ning Bao.
Lu and Geahlen received funding from Purdue and the National Cancer Institute for the study. Lu plans to further develop the technology in the future.
Writer: Douglas M. Main, (765) 496-2050, dmain@purdue.edu
Sources: Chang Lu, 765-494-1188, changlu@purdue.edu
Robert Geahlen, 765-494-1457, geahlen@pharmacy.purdue.edu
Ag Communications: (765) 494-2722;
Beth Forbes, forbes@purdue.edu
Agriculture News Page
PHOTO CAPTION:
A new method developed by Purdue researchers, from left, Chang Lu, Robert Geahlen and Jun Wang allows them to detect movement of proteins within cells, important for cancer cell development and other cell processes. Geahlen holds the microchip through which cells are pumped during the technique. (Purdue Agricultural Communication photo/Tom Campbell)
IMAGE CAPTION:
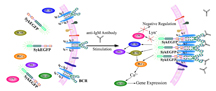
Proteins, labeled SykEGFP, are shown moving from the cell interior to the interior of the plasma membrane. Such movement, or translocation, is involved in cancer development and other important processes. (Purdue image/Chang Lu)
Detection of Kinase Translocations Using Microfluidic Electroporative Flow Cytometry
Directed localization of kinases within cells is important for their activation and involvement in signal transduction. Detection of these events has been largely carried out based on imaging of a low number of cells and subcellular fractionation/Western blotting. These conventional techniques either lack the high throughput desired for probing an entire cell population or provide only the average behaviors of cell populations without information from single cells. Here we demonstrate a new tool, referred to as microfluidic electroporative flow cytometry, to detect the translocation of an EGFP-tagged tyrosine kinase, Syk, to the plasma membrane in B cells at the level of the cell population. We combine electroporation with flow cytometry and observe the release of intracellular kinase out of the cells during electroporation. We found that the release of the kinase was strongly influenced by its subcellular localization. Cells stimulated through the antigen receptor have a fraction of the kinase at the plasma membrane and retain more kinase after electroporation than do cells without stimulation and translocation. We are able to differentiate a cell population with translocation from one without it with the information collected from individual cells of the entire population. This technique potentially allows detection of protein translocation at the single-cell level. Due to the frequent involvement of kinase translocations in disease processes such as oncogenesis, our approach will have utility for kinase-related drug discovery and tumor diagnosis and staging.
To the News Service home page
If you have trouble accessing this page because of a disability, please contact Purdue News Service at purduenews@purdue.edu.
