
| AUDIO |
| • Richard Borgens, director of the Center for Paralysis Research, talks about the significance of hydralazine research. (61 seconds) |
| RELATED INFO |
| • Journal of Neuroscience Research |
| • Center for Paralysis Research |
| • School of Veterinary Medicine |
Purdue scientists find hypertension drug reverses death of cells
WEST LAFAYETTE, Ind. — Purdue University researchers have identified a drug commonly used to treat hypertension that may also reverse damage from spinal cord injuries, cancer and Parkinson's disease.
|
New findings based on research at the cellular level are detailed in two studies published in the Journal of Neuroscience Research today (Monday, April 17). In the first article, researchers examine how acrolein attacks and kills cells. In the second article, they demonstrate that cell death caused by acrolein (a-KRO-le-an), a byproduct of an injury, can be reversed when hydralazine is administered.
"This is probably the most important fundamental discovery we have made at the Center for Paralysis Research because we are saving nerve cells from death," said Borgens, Mari Hulman George Professor of Applied Neurology in the School of Veterinary Medicine and founder of the paralysis research center where the research was conducted.
"Initially we may use this discovery for spinal cord injury and stroke, but we can expect further studies will look at how it works against a whole spectrum of injury and disease," he said.

|
"We analyzed other natural toxins as well, and our success has been remarkable," Borgens said. "We found that more than 80 percent of the cells can be saved with hydralazine."
Acrolein stays in the body for days and is responsible for secondary damage that keeps injured cells from healing. The idea to use hydralazine against acrolein is a logical extension of research on the toxin, such as the use of a beta blocker against high blood pressure or chicken soup for a cold, Shi said.
"Acrolein is one of the causes of free radicals that are known to damage cells, so it makes sense to stop them from ever being produced," said Shi, who is associate professor of basic medical science in Purdue's School of Veterinary Medicine. "With hydralazine, we are attacking the root of the problem rather than the symptom."

|
The Purdue researchers started looking at alternative methods to save cells because other studies that had tried to use antioxidants to deactivate free radical molecules had failed in human clinical trials in traumatic brain injuries, strokes and spinal cord injuries.
"If we intervene early enough, we may have the ability to slow down the process of diseases, such as Alzheimer's and Parkinson's, which would be significant," Shi said. "If we can prevent these diseases from getting worse, we can give people a better quality of life."
Peishan Liu-Snyder, who graduated last summer and will be a post-doctoral fellow at Brown University in June, also was part of the Purdue research team. She became interested in research at the Center for Paralysis Research when it focused on the use of liquid polymers that prevent nerve cells from rupturing, enabling them to heal themselves.
"We found hydralazine works well after the initial injury period because it targets the secondary injury process," said Liu-Snyder. "It binds to the acrolein to inactivate its toxicity."
The research on hydralazine is now in the animal-studies phase. Borgens and Shi have joint appointments in Purdue's School of Veterinary Medicine and the Welden School of Biomedical Engineering.
In the laboratory, hydralazine treatments were added to cell cultures damaged by acrolein, and the deterioration of the nerve fibers was stopped. But hydralazine is not suitable for injury victims because it lowers blood pressure, and it is not likely to be the final solution, Borgens said. Researchers at the Center for Paralysis Research are teaming with department head Stephen Byrn, and Dan Smith, a post-doctoral fellow, both from industrial and physical pharmacy in Purdue's College of Pharmacy, Nursing and Health Sciences, to develop new drugs based on the activity and structure of hydralazine.
"Hydralazine is a remarkable drug, but it's not suitable for cases of traumatic injury where the last thing you want to do is lower blood pressure," Borgens said. "We've embarked on a program now to build a new drug that will do better job than hydralazine and not carry with it any unwanted side effects. We either have to make something completely different or else combat blood-pressure issues with other medications."
One other laboratory, the University of Adelaide in Australia, is studying the effects of hydralazine on natural poisons. While Purdue researchers are looking at hydralazine's effect on acrolein in nerve cells, the Australian lab is concentrating on the molecular mechanisms to determine how it works.
The Center for Paralysis Research was established in 1987 both to develop and to test promising methods of treatment for spinal cord injuries. The center uses its close affiliation with the Department of Veterinary Clinical Sciences in Purdue University's School of Veterinary Medicine to move basic laboratory methods into clinically meaningful veterinary testing.
This research was funded in part by the National Institutes of Health, predoctoral fellowship funds, the State of Indiana, and gifts from Mari Hulman George and Helen Skinner, as well as general funds from Purdue's Center for Paralysis Research.
Writer: Maggie Morris, (765) 494-2432, maggiemorris@purdue.edu
Sources: Richard Borgens, (765) 494-7600, borgens@purdue.edu
Riyi Shi, (765) 496-3018, riyi@purdue.edu
Peishan Liu-Snyder, (765) 494-7600, pliu@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Note to Journalists: Copies of the research papers and video b-roll are available by contacting Maggie Morris at Purdue News Service, (765) 494-2432, maggiemorris@purdue.edu.
PHOTO CAPTION:
Riyi Shi, an associate professor in basic medical sciences and biomedical engineering at Purdue, uses a recording chamber to test the loss of function in a spinal cord sample due to the presence of a toxic chemical called acrolein. Shi, a physician, is with the School of Veterinary Medicine and Weldon School of Biomedical Engineering. (Purdue News Service photo/David Umberger)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2006/shi-acoustics.jpg
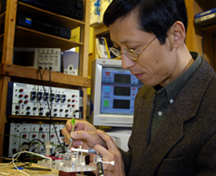
PHOTO CAPTION:
Richard Borgens, professor of basic medical sciences and founder of Purdue University Center for Paralysis Research, discusses new research that identifies a drug commonly used for hypertension that may also reverse damage from spinal cord injuries, cancer and other diseases. (Purdue News Service photo/David Umberger)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2006/borgens-lab.jpg
PHOTO CAPTION:
Peishan Liu-Snyder, a post-doctoral fellow working on research in Purdue University's Center for Paralysis Research, measures the injury current of a spinal cord sample with a vibrating probe. The technique is used in research on the use of a hypertension drug called hydralazine that is able to stop the deterioration of a nerve cell after it is injured by the toxin acrolein. (Photo provided by Center for Paralysis Research/Michel Schweinsberg photo)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2006/peishan-probe.jpg
Acrolein - Mediated Mechanisms of Neuronal Death
and Richard Ben Borgens
It is well known that traumatic injury in the central nervous system can be viewed as a primary injury and a secondary injury. Increases in oxidative stress lead to breakdown of membrane lipids (lipid peroxidation) during secondary injury. Acrolein, an alpha, beta-unsaturated aldehyde, together with other aldehydes, increases as a result of self-propagating lipid peroxidation. Historically, most research on the pathology of secondary injury has focused on Reactive Oxygen Species (ROS) rather than lipid peroxidation products. Little is known about the toxicology and cell death mediated by these aldehydes. In this study, we investigated and characterized certain features of cell death inducted by acrolein on PC12 cells, as well as cells from dorsal root ganglion (DRG) and sympathetic ganglion in vitro. In the companion paper we evaluated a possible means to interfere with this toxicity by application of a compound that can bind to and inactivate acrolein. Here we used both light and atomic force microscopy to study cell morphology after exposure to acrolein. Administration of 100 _M acrolein caused a dramatic change in cell morphology as early as 4 hours. Cytoskelatal structures significantly deteriorated after exposure to 100 _M acrolein demonstrated by fluorescence microscopy while calpain activity increased significantly at this concentration. Cell viability assay indicated significant cell death with 100 _M acrolein by 4 hours. Caspase 3 activity assay and DNA fragmentation assay were performed and supported the notion that 100 _M acrolein induced PC12 cell death by mechanism of necrosis, not apoptosis.
Hydralazine Rescues Pc12 Cells From Acrolein-Mediated Death
Acrolein, a major lipid peroxidation product, has been associated with both CNS trauma and neurodegenerative diseases. Due to its long half-life, acrolein is a potent endogenous toxin capable of killing health cells during the secondary injury process. Traditionally, attempts to intervene in the process of progressive cell death after the primary injury have included scavenging reactive oxygen species (so-called free radicals). The animal data supporting such an approach have generally been positive, while all human clinical trials attempting a similar outcome in human CNS injury have failed. New drugs that might reduce toxicity by scavenging the products of lipid peroxidation present a promising, and little investigated, therapeutic approach. Hydralazine, a well-known treatment for hypertension, has been reported to react with acrolein forming hydrazone in cell-free systems. In the companion paper, we have established an acrolein-mediated cell injury model using PC 12 cells in vitro. Here we test the hypothesis that the formation of hydrazone adducts with acrolein is able to reduce acrolein toxicity and spare a significant percentage of the population of PC 12 cells from death. Using concentrations of approximately 1mM of this aldehyde scavenger can rescue over 80% of the population of PC12 Cells. This study provides a basis for a new pharmacological treatment to reduce the effects of secondary injury in the damaged and/or diseased nervous system. In particular we describe the need for new drugs that possess aldehyde scavenging properties but do not interfere with the regulation of blood pressure.
To the News Service home page