
March 7, 2006
'Shuttling' protein possibly key to resilience of cancer cells
WEST LAFAYETTE, Ind. — Researchers at Purdue University have discovered a molecular mechanism that may play a crucial role in cancer's ability to resist chemotherapy and radiation treatment and that also may be involved in Alzheimer's and heart disease.
|
"Our findings may provide a new avenue for the development of innovative treatments for certain cancers and other conditions," said Chang-Deng Hu, an assistant professor in Purdue's Department of Medicinal Chemistry and Molecular Pharmacology and an investigator at the Walther Cancer Institute in Indianapolis.
The experiments were done using a line of "teratocarcinoma" malignant tumor cells from mice called F9, which, when subjected to the right biochemical signals, have the ability to alter their properties and are considered to be "cancer stem cells." The hypothetical cancer-resistance role of cancer stem cells could explain why tumors return after treatment. If stem cells prove to be critical to cancer's resistance to treatment, new medications might be developed to target cancer stem cells while chemotherapy or radiation is administered, Hu said.
Research findings are detailed in a paper appearing this month in the EMBO Journal, published by the European Molecular Biology Organization. The paper was written by postdoctoral research associate Han Liu, laboratory technician Xuehong Deng and graduate student Y. John Shyu, all in the Department of Medicinal Chemistry and Molecular Pharmacology; Jian Jian Li, an associate professor in the Department of Health Sciences; Elizabeth J. Taparowsky, a professor in the Department of Biological Sciences; and Hu.
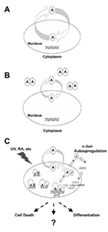
The research focuses on two proteins called c-Jun and ATF2, which are key components of a protein complex called activating protein-1, or AP-1. AP-1 is a major "transcription factor" that binds to DNA, activating the "expression" of genes required to produce proteins needed for vital cellular processes. The proteins that make up AP-1, including ATF2 and c-Jun, often join together in the nucleus, forming either "homodimers," when two of the same proteins join, or "heterodimers," when two different proteins come together.
"Current thinking is that all of these AP-1 proteins in healthy cells are localized, or confined, to the nucleus," Hu said. "But in this work we found for the first time that ATF2 constantly shuttles between the cytoplasm and the nucleus."
The researchers found that the ATF2 protein possesses "nuclear export" and "nuclear localization" signals, which enable it to travel out of and back into the nucleus, respectively. The researchers also discovered that if ATF2 attaches to c-Jun in the nucleus, forming a heterodimer, the nuclear export signal is blocked, preventing ATF2 from traveling from the nucleus to the cytoplasm.
Researchers had already known that chemotherapy and radiation cause cancer cells to increase production of ATF2. The Purdue researchers found that "overexpressed," or overproduced, ATF2 is predominantly located in the cytoplasm because of an inadequate amount of c-Jun in the nucleus, suggesting it is likely that overexpressed ATF2 also may be localized in the cytoplasm in cancer cells, Hu said.
The Purdue researchers not only discovered that ATF2 is localized in the cytoplasm of the mouse cancer stem cells, but also that exposing the cells to ultraviolet light induced more production of c-Jun protein in the nucleus, causing the ATF2 to bind with c-Jun, stopping the shuttling process and causing cell death. The c-Jun-ATF2 heterodimers cause more c-Jun protein to be produced, attracting more ATF2 and reinforcing the localization of ATF2 in the nucleus.
Because it has been reported that ATF2 overexpression causes the resistance of cancer cells to chemotherapy and radiation, the ATF2 shuttling might play a key role in the ability of cancer cells to resist cancer treatments, and preventing the ATF2 from moving into the cytoplasm might improve the effectiveness of anticancer treatments.
"Ultimately, we are trying to figure out how to make the cancer cells more sensitive to chemotherapy and radiation treatment by keeping the ATF2 in the nucleus," Hu said.
The Purdue researchers tracked ATF2 and other proteins using a fluorescent imaging technique developed by Hu called bimolecular fluorescence complementation. The procedure involves breaking a fluorescent protein into two fragments and then fusing each fragment to different AP-1 proteins, including c-Jun and ATF2. When the proteins later bind to form a heterodimer, the fluorescent-protein fragments are reunited, causing them to glow green when illuminated with a light source. The fluorescence enabled the researchers to pinpoint the location of c-Jun-ATF2 heterodimers and discover the shuttling movements of ATF2. The ATF2 protein also has been found in the cytoplasm of diseased brain cells in Alzheimer's disease and muscle cells in the heart, suggesting the same shuttling mechanism might be involved in those conditions.
"These findings suggest a new avenue to study how ATF2 is implicated in the pathogenesis of these diseases," Hu said.
Hu and co-authors Li and Taparowsky are affiliated with the National Cancer Institute-designated Purdue Cancer Center and with the Oncological Sciences Center in Discovery Park, the university's hub for interdisciplinary research. The research has been funded by the Purdue Cancer Center, Indiana Elks Charities Inc., Walther Cancer Institute, National Institutes of Health and National Science Foundation.
Writer: Emil Venere, (765) 494-4709, venere@purdue.edu
Sources: Chang-Deng Hu, (765) 496-1971, cdhu@pharmacy.purdue.edu
Jian Jian Li, (765)496-6792, jjli@purdue.edu
Elizabeth J. Taparowsky, (765)494-7978, ejt@bilbo.bio.purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Note to Journalists: The research paper has been posted online.
ILLUSTRATION CAPTION:
This diagram depicts how a protein called ATF2 constantly shuttles from the nucleus to the cytoplasm in cells, a mechanism that may play a crucial role in cancer's ability to resist chemotherapy and radiation treatment and that also may be involved in Alzheimer's and heart disease. ATF2 was previously believed to be confined to the nucleus of healthy cells, but researchers at Purdue University discovered that it actually shuttles between the nucleus and cytoplasm. The researchers used a novel imaging technique invented at Purdue to track the protein, learning that its shuttling is controlled by the presence of another protein in the nucleus and its attachment to that second protein. The findings could provide a new avenue for the development of innovative treatments for certain cancers and other conditions. (Purdue Department of Medicinal Chemistry and Molecular Pharmacology)
A publication-quality photo is available at https://www.purdue.edu/uns/uns/images/+2006/hu-shuttling.jpg
Mutual regulation of c-Jun and ATF2 by transcriptional activation and subcellular localization
1
Department of Medicinal Chemistry and Molecular Pharmacology,Purdue University
2School of Health Science, Purdue University
3Department of Biological Sciences, Purdue University
4Purdue Cancer Center
5Walther Cancer Institute, Indianapolis, IN
ATF2 and c-Jun are key components of activating protein-1 and function as homodimers or heterodimers. C-Jun—ATF2 heterodimers activate the expression of many target genes, including c-jun, in response to a variety of cellular and environmental signals. Although it has been believed that c-Jun and ATF2 are constitutively localized in the nucleus, where they are phosphorylated and activated by mitogen-activated proteins kinases, the molecular mechanisms underlying the regulation of their transcriptional activities remain to be defined. Here we show that ATF2 possesses a nuclear export signal in its leucine zipper region and two nuclear localization signals in its basic region, resulting in continuous shuttling between the cytoplasm and the nucleus. Dimerization with c-Jun in the nucleus prevents the export of ATF2 and is essential for the transcriptional activation of the c-jun promoter. Importantly, c-Jun-dependent nuclear localization of ATF2 occurs during retinoic acid-induced differentiation and UV-induced cell death in F9 cells. Together, these findings demonstrate that ATF2 and c-Jun mutually regulate each other by altering the dynamics of subcellular localization and by positively impacting transcriptional activity.
To the News Service home page