 Purdue News
Purdue News
 Purdue News
Purdue News
|
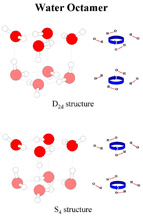
In addition, these tiniest cubes came in two forms, which had the same mass and structure, but differed in the arrangement of the hydrogen bonds within the cubes.
The study, which appears in the June 13 issue of the journal Science, was conducted by chemist Timothy Zwier, postdoctoral associate Caleb Arrington, and graduate students Christopher Gruenloh and Joel Carney.
"These findings verify what theorists have predicted for years; namely, that the eight water molecules preferentially form a cubic structure," Zwier says. "It also provides the first evidence that even in very small water clusters, water has the capacity to arrange its hydrogen bonds in several distinct orientations, much as it does in forming the many different solid phases of ice."
The study gives the first glimpse of nature's tiniest ice cubes and provides new information on the unique ability of water to hydrogen bond to itself to form large networks. This ability gives water many of its unique properties, including the unusual capacity of solid ice to float on liquid water.
Water, known as the "universal solvent," also has the unusual ability to dissolve a variety of substances. "Our understanding of water is so important because all biological processes, including those occurring in the human body, take place in a water-based solution", Zwier says.
What makes water unique are the hydrogen bonds that form between water molecules and other molecules. Each V-shaped molecule of water contains one oxygen atom centered between two hydrogen atoms. The molecule is held together by chemical bonds that create a slightly negative charge on the oxygen atom and a small positive charge on each of the hydrogen atoms.
This unequal charge distribution makes the water molecule extremely "sociable", eager to bond with other water molecules, and gives water its unequaled ability to dissolve compounds.
To analyze how these hydrogen bonds form in small water clusters, Zwier and co-workers used a high-pressure gas expansion to cool water molecules in the gas phase to temperatures as low as 1 degree Kelvin, the equivalent of -457 degrees Fahrenheit.
As the water cooled and condensed into solid clusters, some of the clusters incorporated a single benzene molecule on their surface. The benzene molecule allowed the various clusters to be identified by size using lasers to "weigh" the clusters.
Once he identified the cubic clusters of eight, Zwier and his colleagues applied an infrared laser to excite the clusters, causing the hydrogen bonds in the tiny cubes to stretch and contract. By analyzing the wavelengths of this spectrum, he was able to determine the molecular arrangement of the hydrogen bonds within the cubes.
He found that the cubes were identical in mass and structure, with each cube made up of four molecules of water stacked on top of the other four molecules. Though the hydrogen bonds in the top layer of each cube were oriented in the same manner, the hydrogen bonds in the bottom layers of the cubes took one of two possible arrangements, with the bonds facing either the same direction or opposite direction as the bonds in the top layer of the cube.
"Since the two structures are virtually identical in energy, the orientation that a particular cluster takes depends on the specific collisions the cluster undergoes while it is being made," Zwier says.
"It is interesting that already with only eight water molecules, water makes up two different 'phases' which differ only in the orientations of the hydrogen bonds," he says. "This is the beginnings of what we know to be true in the solid phase. Water has more solid phases -- nine total -- than any other known pure substance because it can form phases which differ only in the orientations of the hydrogen bonds."
CONTACT: Zwier, (765) 494-5278; e-mail, zwier@chem.purdue.edu
Writer: Susan Gaidos, (765) 494-2081; e-mail, susan_gaidos@purdue.edu
Purdue News Service: (765) 494-2096; e-mail, purduenews@purdue.edu
Photo caption
Color photo, electronic transmission, and Web and ftp download available. Photo ID:
Zwier/water
Download here.