
Runrun Wu

Training Group:
Biomolecular Structure and Biophysics
Mentor / Lab:
Dr. Nicholas Noinaj
Specific Research Area / Project:
Structural and Functional Studies of β-barrel Assembly Machinery
Lab / Personal work-related websites:
LabResearch Profile:
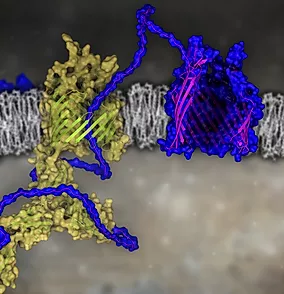
The OMPs from Gram-negative bacteria have a membrane spanning domain that is composed of 8-36 β-strands into a β-barrel fold.These OMPs are essential for adhesion, signaling, and nutrient import. OMPs are first synthesized in the cytoplasm and transported into the periplasm through the Sec translocation machinery. The biogenesis of these OMPs is mediated by BAM, a multicomponent complex. In E. coli, BAM is composed of five components, including BamA (88 kDa), BamB (40 kDa), BamC (34 kDa), BamD (26 kDa), and BamE (11 kDa). As the central component of the BAM complex, BamA is an OMP itself and serves as the scaffold to assemble the complex and orchestrate OMP biogenesis. BamB-E are lipoproteins that anchored into the periplasmic leaflet of the outer membrane.Decades of structural and functional studies have shown that BamA and BamD are essential for cell survival, thus are considered as the core component of the complex. Although BamB, BamC and BamE are considered accessory, cells that lack these components are less likely to survive in otherwise harsh conditions during an infection.Despite that recent structural and functional studies have collectively advanced our understanding of the BAM complex, the native structure of BAM in its lipid environment remains unknown, which may otherwise shed light on the assembly of BAM in vivo. Also, the underlying mechanism on how BAM recognize OMPs and sort them into the outer membrane is ambiguous. Therefore, the goal of my research is to decipher the structure of BAM in its near-native environment, and understand the mechanism of BAM in regulating OMP biogenesis.
About Me:
Started as a chemist, I grew more and more interested in life science, and that's why I joined Purdue. PULSe provides a good platform for me to begin my journey in life sciences, and allows me to transit without much pain since the rotating experience really prepares me for the lab I am joining now.
With my PhD degree earned, I see myself combining my chemistry and structural biology knowledge together, and working on infectious diseases in the future, which is a growing issue concerning human health.
Awards:
- Helmsley Fellowship from The Helmsley Charitable Trust
- Lynn Fellowship, Purdue University, USA (2017)
Publications:
- Wu, R., Stephenson, R., Gichaba, A. and Noinaj, N., 2020. The big BAM theory: An open and closed case?. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1862(1), p.183062.
- Imai, Y., Meyer, K.J., Iinishi, A. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019).
Presentations:
- Wu, R., Klose, T., & Noinaj, N. Characterization of the β-barrel Assembly Machinery in Nanodiscs using CryoEM, ACA 2019 annual meeting, Covington, KY, USA
- Wu, R., & Noinaj, N. Characterization of the β-barrel Assembly Machinery in Nanodiscs using CryoEM, 14 th Midwest Conference on Protein Folding, Assembly and Molecular Motions, University of Notra Dame, IN, USA
- Wu, R., & Noinaj, N. Characterization of the β-barrel Assembly Machinery in Nanodiscs using CryoEM, Missouri Symposium in Molecular Biophysics, Columbia, MO, USA
- Wu, R., & Noinaj, N. Characterization of the β-barrel Assembly Machinery in Nanodiscs using CryoEM, PULSe spring reception, Purdue University, West Lafayette, IN, USA.
- Wu, R., Liu, Y., & Rossmann, M. 3D structure of horse spleen apoferritin determined by cryo-EM. First year poster session 2017, Purdue University, West Lafayette, IN, USA.
- Oral Presentation:
- Wu, R. Cryo-EM studies of the BAM complex. Midwest Consortium PI Meeting, 2020, Purdue University, West Lafayette, IN, USA.
- Student Profiles
- Aktan Alpsoy
- Andrew Asberry
- Carlos A. Brito-Sierra
- Clairissa Corpstein
- Hao Chen
- Rachel Foguth
- Logan Ganzen
- Veronica Heintz
- Ethan Hillman
- Kathryn Jacobson
- Steven McKenzie
- Vinay Menon
- Jasmine Moore
- Alexandr Pak
- Raquel Peron
- Runrun Wu
- Sudhanshu Shekhar
- Janiel Ahkin Chin Tai
- Yu Tang
- Chelsea Theisen
- Samantha Tinsley
- Nicole Vike
- Ravi Yadav

