
PULSe Faculty Profiles
Faculty affiliated with the Institute for the Sustainable Future and the ESE program include over 100 researchers from across campus, all of which are interested in interdisciplinary research and education on environmental topics. Below we show faculty currently affiliated with the program. There are many unique and emerging issues in environmental sciences that faculty are pursuing.
| Last Name | First Name | Training Group(s) | |
|---|---|---|---|
| Aguilar | Ruben | Cancer Biology, Integrative Neuroscience, Membrane Biology |
Ruben AguilarAssistant Professor, Department of Biological Sciences Current Research InterestsMy laboratory is focused in the study of protein trafficking and membrane transport in relation to the processes of cell polarity establishment (a feature that is key for animal development and crucial for physiological functions such as synaptic transmission and immune response) and carcinogenic transformation. In order to pursue our research goals we routinely use genetic, biochemistry and cell biology techniques with yeast and mammalian cells. We study protein-protein interactions at molecular level by using bioinformatics, biochemical and genetic tools (like the two-hybrid system) and we investigate the physiological relevance of these interactions by using functional assays, microscopy (of live and fixed cells) and genetic approaches.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactraguilar@purdue.edu  |
| Allen-Petersen | Brittany | Cancer Biology |
Brittany Allen-PetersenAssistant Professor Current Research InterestsProtein phosphatases predominantly function as gatekeepers to kinase signaling, providing the necessary “off-switch” to prevent uncontrolled growth. Cancer cells, however, can develop mechanisms that suppress phosphatase function. My research program focuses on understanding the phosphatase-dependent signaling events that contribute to cellular plasticity and the impact of these signals on pancreatic cancer initiation and progression. Currently, there are two main areas of research in my lab: 1) Determining the consequence of phosphatase deregulation on cancer cell fate and therapeutic resistance and 2) Exploring the therapeutic potential of phosphatase activators in complex tumor models. These studies utilize a variety of cellular- and molecular- biology techniques, including 3D culture and in vivo cancer models.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactballenpe@purdue.edu  |
| Altman | Ryan | Chemical Biology, Integrative Neuroscience, Microbiology, Immunology and Infectious Diseases |
Ryan AltmanAssistant Professor Current Research InterestsFluorinated Compounds in Medicinal Chemistry The incorporation of a fluorinated substituent on a target molecule typically alters many physicochemical and pharmacokinetic properties related to the design of therapeutics. For instance, fluorination typically alters hydrophilicity and lipophilicity, electrostatic and electronic properties, metabolic, thermal and oxidative stability, conformational rigidity, acid/base properties, and binding interactions between the small molecule and the biological target. Because of these perturbations, the ability to access fluorinated compounds is critical for developing new therapeutics and agrochemical agents, and as a results of these annually 35–45% of FDA approved therapeutics bear at least one fluorine atom. Within this field, the Altman Group develops innovative reactions, reagents and synthetic strategies for accessing medicinally relevant fluorinated functional groups. Further, the employs its own synthetic transformations, as well as innovative synthetic reactions from others, to access new biological probes and therapeutic candidates with improved drug-like properties. Fluorinated Peptidomimetics for Delivering Peptides into the Central Nervous System Endogenous opioid peptides regulate activity within the central nervous system (CNS), and are particularly interesting for treating pain, depression, and anxiety. Unfortunately, clinical use of peptide-based agents is restricted by poor physicochemical and biophysical properties, which limit penetration into the CNS. Therefore, many peptide-based probes cannot be employed clinically for treating many disease states. To address this problem, the Altman group explores the use of fluorinated peptidomimetics (FPMs) to improve the drug-like properties of peptides, and to deliver peptides into the CNS. Recent efforts have provided rationally designed orally bioavailable FPM-based analogs of opioid peptides that cross the blood-brain-barrier. To access these unique target molecules, the group has developed new synthetic methods and strategies, which should be broadly applicable for accessing FPMs to address many disease states. The target FPM molecules are typically subjected to several in vitro and in vivo assays to evaluate pharmacodynamic, antinociception, distribution, metabolism, and pharmacokinetic properties. Data from the study will used to develop computational models to predict opioid activity and drug-like properties, which facilitates the design of new analogs. This overarching strategy should be amenable for modulating physicochemical and biophysical properties of a broad spectrum of neuropeptides, with the ultimate goal of converting small peptide-based probes into CNS-active clinical candidates. To support this project, the Altman group collaborates with the Van Rijn Group (Purdue University) whose pharmacological expertise complements our distribution, metabolism and pharmacokinetic goals. Regulating the Kynurenine Pathway The kynurenine pathway (KP) regulates tryptophan metabolism and generates many modulatory biomolecules that in turn directly correlate to affect various aspects of neurotransmission, neurotoxicity, neuroprotection, inflammation, and other immunological functions. Further, dysregulation of this pathway directly correlates to many disease states, including neurological disorders, infectious diseases, and cancer, thus making small molecule modulators of the KP critical for understanding the diseases states, and for providing potential therapies. Within this area, the Altman group works collaboratively to develop small molecule probes for studying and modulating enzymes in the kynurenine pathway. In some cases, these probes are used to study unique aspects of KP enzymology, while other efforts aim to develop small-molecule probes for modulating in vitro and in vivo models of various disease states. Long-term, these biological probes might serve as leads for downstream medicinal chemistry optimization. To support this project, the group actively collaborates with the research groups of Prof. Aimin Liu (UTSA) whose groups bring expertise in biochemistry and immunology to the project.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactraaltman@purdue.edu  |
| Andrisani | Ourania | Cancer Biology |
Ourania AndrisaniProfessor of Basic Medical Sciences Current Research InterestsMy research interests and expertise are on molecular mechanisms of transcriptional regulation, epigenetics, and signal transduction involved in cell growth control, cellular differentiation and cancer pathogenesis. My laboratory studies epigenetic mechanisms involved in Hepatitis B virus (HBV) biosynthesis and virus-mediated hepatocarcinogenesis. Our ongoing studies focus on cellular factors regulating both virus biosynthesis and formation of hepatic cancer stem cells (hCSCs). Our goal is to identify essential mechanisms that can be targeted to suppress HBV infection and the resulting HBV-mediated liver cancer. One such mechanism identified by our studies is activation of the cellular S/T kinase Polo-like-kinase 1 (Plk1) by the virus-encoded oncogenic HBx protein. We have shown that Plk1 activation exerts a crucial role both as a positive effector of HBV replication and HBV-mediated oncogenic transformation. In collaboration with the team of Professor Philippe Merle, M.D., Ph.D., Medical Co-Director of the Liver Department at Lyon University Hospital, France, we have shown that HBV infection, via Plk1, deregulates an epigenetic mechanism that alters stability and function of the Polycomb Repressive complex 2 (PRC2). This deregulation involves downregulation of the RNA helicase DDX5, which interacts with PRC2 and the noncoding RNA HOTAIR to repress transcription of specific genes. Interestingly, we have identified re-expression of select DDX5/PRC2 repressed genes in liver tumors associated with poor patient prognosis. These genes include markers of hCSCs and pluripotency genes, referred to as the hCSC-like gene signature. Importantly, we have evidence that DDX5 becomes downregulated, via HBV-mediated induction of two oncomiRs, miR17-92 and miR106b-25. Interestingly, HBV replicating cells with knockdown of DDX5 exhibit resistance to chemotherapy drugs, including sorafenib. Sorafenib is the only available treatment for advanced liver cancer but it only prolongs patient life by few months, because of resistance. Our ongoing studies have identified a likely mechanism contributing to sorafenib insensitivity of the DDX5-knockdown cell lines, which involves escape from a non-apoptotic cell death called ferroptosis. Our goal is to test this hypothesis, and develop a preclinical model of liver cancer to interfere with this pathway in vivo. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactandrisao@purdue.edu  |
| Applegate | Bruce | Microbiology, Immunology and Infectious Diseases |
Bruce ApplegateAssistant Professor Food Science Current Research InterestsMy research interests include detection of viable foodborne pathogens using bateriophage, automated extraction of nucleic acids from various matrices, enumeration of microorganisms using quantitative PCR, the use of bioreporters in bioelectronics, metabolic engineering, detection of problematic microorganisms in industrial environments, construction of recombinant bacterial strains to rapidly evaluate antimicrobial products, and microbial ecology. Patents Applegate, B. M., Morgan, M., Perry, L., & Kothapalli, A. (2009). Methods for Generation of Reporter Phages and Immobilization of Active Bacteriophages on a Polymer Surface. U.S. Patent No. Filed Utility Patent # 12/549,500.. Washington, D.C.: U.S. Patent and Trademark Office. Applegate, B. M. (2005). Bioluminescent biosensor device. U.S. Patent No. 6,544,729. Washington, D.C.: U.S. Patent and Trademark Office. Applegate, B. M., Morgan, M., Perry, L., & Kothapalli, A. (2009). Methods for Generation of Reporter Phages and Immobilization of Active Bacteriophages on a Polymer Surface. U.S. Patent No. Filed Utility Patent # 12/549,500.. Washington, D.C.: U.S. Patent and Trademark Office. Applegate, B. M. (2005). Bioluminescent biosensor device. U.S. Patent No. 6,544,729. Washington, D.C.: U.S. Patent and Trademark Office. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactapplegab@purdue.edu  |
| Axelrod | Abram | Chemical Biology, Microbiology and Infectious Diseases |
Abram AxelrodAssistant Professor of Chemistry
Current Research InterestsOur current research interests are concerned with the preparation of molecules relevant to immunology and oncology, and using them as tools in medicinal chemistry and pharmacology. Often referred to as 'biologics’, glycoproteins are now an established class of therapeutic macromolecules utilized in medicine. Our laboratory is actively involved in the synthesis of single isoform, glycosylated peptides. Representative projects include the design of ovarian cancer-related epitopes utilized for antibody generation against aggressive tumors, the synthesis of hybrid diabodies capable of macrophage reprogramming in both cancer and autoimmune diseases, and preparing disease-specific targeted FOXO transcription factors as new types of therapeutics. As an extension of our interest in the synthesis and chemical biology of glycoproteins, we are focusing on developing new catalytic amide bond-forming reactions as part of this program. Oligosaccharides mediate complex signaling events in the body, and demonstrate manifold biological activities. We are investigating the synthesis of unusual carbohydrate constructs with potential to invigorate stalled immune responses in individuals with chronic infections and cancer. We are specifically focusing on the activation of dendritic cells, natural killer (NK) cells, and T cells though auxiliary stimulation mechanisms, independent of major receptor-induced activation. By developing concise platforms to biologically active, structurally-complex natural products, we can enable more rapid investigation into their pharmacology and potential therapeutic development. Our laboratory is pursuing the synthesis of both immunomodulatory and anti-cancer molecules possessing novel mechanisms of action, with an emphasis on pancreatic and lung cancers. Importantly, our approaches to these natural products will be able to produce edited analogues for identifying structure-activity relationships and be capable of incorporating radiolabeled probes for imaging and diagnostic purposes. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactaaxelro@purdue.edu
 |
| Banks | Jo Ann | Plant Biology |
Jo Ann BanksProfessor Current Research InterestsCoordinator of the Selaginella genome sequence and its comparison to other plant genomes. See website: http://rna.genomics.purdue.edu/Archived_Items/Agry_600/Smo5_project for more information Molecular basis of arsenic hyperaccumulation in the fern Pteris vittata. This fern is amazing as it tolerates and accumulates a lot of arsenic in its fronds. We will continue to discover and study the genes that are involved in this unusual trait. Sex determination by pheromones in plants and the fern Ceratopteris in particular. Ceratopteris gametophytes develop as hermaphrodites or males, a decision dictated by a pheromone. We are using genetics and genomics approaches to understand how this pheromone regulates this important decision. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactbanksj@purdue.edu  |
| Bartlett | Edward | Computational and Systems Biology, Integrative Neuroscience, Membrane Biology |
Edward BartlettProfessor, Departments of Biological Sciences and Biomedical Engineering Current Research Interests1) Neural circuitry and information processing in sensory systems, especially auditory neural circuitry. 2) Neurophysiology using multichannel recordings, brain slice recordings, auditory evoked potentials. 3) Changes in hearing due to aging, blast injury, or noise exposure. This includes diagnostics, time course and mechanisms of change, and testing therapeutic programs. 4) Computational models of thalamocortical and auditory circuits at the single cell and network levels. 5) Alteration of information processing in neural circuits due to circuit manipulation, such as infrared laser stimulation, optogenetic modification, or chemogenetic modification. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactebartle@purdue.edu  |
| Bharadwaj | Hari | Integrative Neuroscience |
Hari BharadwajAssistant Professor, Department of Speech, Language, & Hearing Sciences, and Weldon School of Biomedical Engineering Current Research InterestsThe Systems Neuroscience of Auditory Perception lab (SNAPlab) is directed by Hari Bharadwaj, and is part of the Department of Speech, Language & Hearing Sciences, and the Weldon School of Biomedical Engineering. At SNAPlab, we study the biological computations and neural circuits that underlie auditory perception. In particular, we are interested in how we process sounds and analyze acoustic scenes in complex everyday environments. Complex scenes with multiple sound sources such as crowded restaurants and busy streets present unique challenges for both biological and machine audition. SNAPlab integrates behavioral experiments, computational modeling, and an array of non-invasive physiological measurement and imaging tools to investigate these challenges in humans.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthbharadwaj@purdue.edu
 |
| Bhunia | Arun | Microbiology, Immunology and Infectious Diseases |
Arun BhuniaProfessor of Molecular Food Microbiology Current Research InterestsMicrobial pathogenesis, host immune response, and bioengineered probiotics approach in mitigating foodborne pathogen infection.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactbhunia@purdue.edu  |
| Boavida | Leonor | Plant Biology |
Leonor BoavidaAssistant Professor Current Research InterestsCell and Developmental Biology; Cell-Cell Signaling; Gamete Biology and Plant Fertilization The union of a sperm and an egg is one of the most remarkable examples of cell-cell recognition: upon membrane contact, two haploid sex cells or gametes fuse to create a brand new organism. In flowering plants, the occurrence of two separate gametic fusions (double fertilization) add another level of complexity to the cellular interactions that happen during fertilization: twin sperm cells fuse with distinctive efficacy with each of the female gametes, the egg and the central cell to form the zygote and the nourishing endosperm. This means that flowering plants evolved a new set or adapted a pre-existing signal transduction machinery to ensure the precise fusion of two pairs of gametes. However, the molecular partners and signaling pathways that regulate gamete interactions remain unclear. The research in our lab seeks to understand the cellular and molecular basis of plant fertilization. To achieve this goal we use a variety of experimental techniques including fluorescent activated cell sorting, advanced live cell imaging, genetic, molecular and functional genomic tools. Current projects in the lab include: (1) determining the role of Tetraspanin-associated membrane microdomains in gamete function; (2) identifying signaling pathways regulating gamete interactions and double fertilization. This work aims to fulfill our quest to understand basic principles that regulate a fundamental biological process that sustain life and plant diversity. We will then be able to apply this knowledge to develop novel and more efficient strategies aimed to manipulate plant fertilization, introduce desirable reproductive traits or increase and stabilize crop productivity. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactlboavida@purdue.edu  |
| Bolin | Jeffrey | Biomolecular Structure and Biophysics, Chemical Biology |
Jeffrey BolinProfessor of Biological Sciences Associate Vice President for Research Current Research InterestsOur research centers on defining relationships between the three-dimensional structures of proteins and their functions through the application of X-ray crystallography in combination with other biophysical and biochemical methods. We focus on studies of metalloenzymes because of their special abilities to catalyze difficult reactions. One current project involves enzymes involved in the biodegradation of aromatic and haloaromatic compounds, a process that has potential applications in the bioremediation of deleterious pollutants. To facilitate adaptation of microbial pathways to this use, as well as to understand the chemistry of the reactions involved, we are investigating the enzymes involved in the degradation of biphenyl and polychlorinated biphenyls (PCBs). We recently determined the crystal structure of the extradiol dioxygenase that catalyzes the critical ring-cleavage step in the degradation of biphenyl and PCBs; this first high resolution structure of any extradiol dioxygenase led to detailed studies of the evolution of homologous enzymes of both similar and divergent functions. Current efforts target enzymes that catalyze other steps in the same and related pathways. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactjtb@purdue.edu  |
| Bowers | Laura | Cancer Biology |
Laura BowersAssistant Professor University of Texas at Austin
Current Research InterestsMy research program focuses on investigating the role of chronic adipose tissue inflammation and other factors in the link between obesity and breast and colon cancer. There are currently two major tracks in my program. The first track will determine whether inflammation-promoting changes in the gut microbiota play a key role in the link between obesity and elevated colon cancer risk. The second is examining whether leptin-induced cancer stem cell enrichment mediates obesity-associated breast cancer chemoresistance. Through enhanced understanding of the mechanisms mediating the obesity-cancer link, we aim to discover: 1) Biomarkers that will help identify the patients at highest risk of cancer development and mortality within the obese population, 2) Novel dietary and pharmaceutical interventions that reduce the impact of obesity on cancer risk and outcomes in these patients.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact765-496-0143
Stone Hall 206
 |
| Bowman | Aaron | Integrative Neuroscience |
Aaron BowmanProfessor and Head of the School of Health Sciences Current Research InterestsAaron Bowman is Professor and Head of the School of Health Sciences and member of the Purdue Institute for Integrative Neuroscience (PIIN). His lab utilizes a combined approach of molecular genetics, pharmacology, biochemistry, cell and developmental biology to understand the role of gene-environment interactions between metal exposure and neurodevelopmental and neurodegenerative diseases including Parkinson's Disease, Alzheimer's Disease and Huntington's Disease. The lab employs a diverse range of model systems including patient-derived induced pluripotent stem cells (iPSCs), neuronal cultures and mouse models. The lab aims to define mechanisms of neuronal dysfunction and understand the basis of selective neuropathology, by characterizing the molecular function of disease genes and their interaction with environmental toxicants under both normal and pathological conditions. The lab has been applying iPSC technology to neurotoxicity research for 13+ years, and has established protocols to generate iPSC lines and differentiate them down forebrain, striatal and midbrain neural lineages. The lab applies toxicological and functional approaches, in addition to novel high-throughput screening and high-content approaches. The long-term goal is to use a personalized medicine approach to investigate patient-specific toxicant vulnerabilities and develop neuroprotective strategies that mitigate neurological diseases with environmental etiologies. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Brewster | Amy | Integrative Neuroscience |
Amy BrewsterAssistant Professor Current Research InterestsMy general research interests are to study mechanisms that underlie molecular and structural dendritic alterations, seizures, and behavioral deficits in epilepsy. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactabrewst@purdue.edu  |
| Briggs | Scott | Cancer Biology |
Scott BriggsAssociate Professor of Biochemistry Current Research InterestsChromatin and histone modifications: In the eukaryotic cell, the precise organization and regulation of chromatin is critical for many cellular processes such as transcription, replication, recombination, repair, and chromosomal segregation. Although chromatin is defined as DNA associated with proteins, the fundamental repeating unit of chromatin is the nucleosome. The nucleosome consists of two copies of each core histone protein (H3, H4, H2A, and H2B) and 146 base pairs of DNA that wraps twice around them. Histone proteins contain a central histone-fold domain and N- and C-terminal tail domains that are subjected to extensive posttransitional modifications. Since posttransitional modifications on histones such as acetylation, phosphorylation, ubiquitination, and/or methylation can influence the chromatin environment and ultimately gene expression, we are interested in studying the enzymes and their associated proteins that mediate these modifications and how misregulation of these enzymes can lead to a disease state. Histone methyltransferases and Cancer: Many SET domain-containing proteins have been associated with human cancers suggesting that they play an important regulatory roll in the cell. However, only a few have been identified as histone methyltransferases such as MLL1 and EZH2. Many of these SET domain-containing proteins are found either mutated, chromosomal translocated, or over-expressed when isolated from oncogenic cells. Therefore, we are interested in determining how mis-regulation and/or aberrant expression of these methyltransferases can lead to an oncogenic event and how aberrant histone methylation may play a role in oncogenesis . Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsdbriggs@purdue.edu  |
| Cannon | Jason | Integrative Neuroscience |
Jason CannonAssociate Professor of Toxicology Current Research InterestsWe are a neurotoxicology laboratory focused on identifying mechanisms adverse neurological and mental health outcomes resulting from environmental exposures. We have primarily focused on Parkinson’s disease, but are also interested in overlapping pathways between other neurological diseases and mental health disorders. We aim to use our mechanistic neurotoxicology data to identify exposures that should be reduced and also to test new therapeutic targets. We are particularly interested in pathogenic interactions between genetic factors and environmental insults. Ongoing projects are primarily focused on the neurotoxicity of 1) heterocyclic amines, which are formed during high temperature meat cooking and; 2) per- and polyfluoroalkyl substances (PFAS), synthetic compounds found in the blood of nearly all humans. Mechanistic studies examine the pathogenic pathways that underlie neurotoxicity. The major techniques utilized in the lab are neurobehavioral analysis, neurochemistry (HPLC w/electrochemical detection) and histopathology/microscopy using a variety of in vitro and in vivo systems. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcannon6@purdue.edu  |
| Carroll | Chad | Biotechnology |
Chad CarrollAssistant Professer Current Research InterestsThe long-term goal of our research program is determine the molecular mechanisms contributing to the development of tendinopathies in order to develop effective treatment strategies. We are determining the mechanisms responsible for the increase in tendinopathy risk in diabetic patients and older adults. We are particularly interested in: 1) the role of advanced glycation end-products in the development of diabetic tendinosis, 2) the role of estrogen in tendon health, and 3) how a combined nutrition/exercise approach can improve tendon properties in older adults. My laboratory uses both human and rodent models to address our research goals in a bench-to-bedside manner. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcarrol71@purdue.edu
 |
| Chan | Deva | Biotechnology, Integrative Neuroscience |
Deva ChanAssistant Professor Current Research InterestsSoft tissue biomechanics and extracellular matrix biology, mechanobiology of hyaluronic acid synthesis, and magnetic resonance imaging applied to musculoskeletal and neural biomechanics.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdevachan@purdue.edu  |
| Chang | Henry | Cancer Biology, Membrane Biology |
Henry ChangAssistant Professor, Biological Sciences Current Research InterestsIt is now clear that endocytosis is much more than cells "drinking" and "eating", but can directly regulate the activities of signaling molecules important for cell-cell communication. To understand this process further, we have focused on dissecting the roles of clathrin-mediated endocytosis in the Drosophila Notch pathway. The Notch pathway is a signaling cascade highly conserved in all metazoans, and has been implicated in a variety of developmental processes. Genetic data from several systems have suggested that, in the Notch pathway, endocytosis has an unusual role of activating the receptor by internalizing its ligand. Still, how this is accomplished remains poorly understood. We have identified mutants defective in endocytosis and in Notch signaling, and we are using a combination of molecular genetics and high-resolution microscopy to understand the functions of these genes. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contacthcchang@purdue.edu  |
| Chang | Leifu | Biomolecular Structure and Biophysics, Cancer Biology |
Leifu ChangAssistant Professor, Department of Biological Sciences
Current Research InterestsCryo-EM, a technique that wins Nobel Prize in Chemistry 2017, has become a mainstream for structure determination of macromolecular complexes due to a recent resolution revolution. Dr. Chang combines cryo-EM and biochemical reconstitution approach to understand the molecular mechanism of large protein complexes, focusing on those in cell cycle regulation. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactHOCK 129  |
| Chapple | Clint | Plant Biology |
Clint ChappleProfessor of Biochemistry Current Research InterestsThe role of the Mediator complex in the regulation of carbon allocation to phenylpropanoid metabolism The sun is the principle source of energy for our planet, and photosynthesis is the primary mechanism by which that energy is captured and stored in the form of reduced carbon. An outcome of these biochemical events is that plants represent a quantitatively important, sustainable, and carbon-neutral source of energy for humans. In order to maximize the utility of plants for this purpose, it is important that we gain control of the processes associated with energy capture and storage, including the molecular mechanisms that allocate fixed carbon to the myriad biochemical pathways in plants. One of the most significant of these is the phenylpropanoid biosynthetic pathway which leads to the deposition of lignin. Lignin is a cross-linked phenolic polymer that makes the cell walls of specialized plant cells more rigid. Its synthesis represents the single largest metabolic sink for phenylalanine in the biosphere and as such represents a huge metabolic commitment for plant metabolism. Lignin is also a significant barrier to the use of crops for livestock feed, pulp and paper production, and to the generation of cellulosic biofuels. Our objective is to push forward our basic understanding of lignin biosynthesis while simultaneously adding to our ability to engineer plant metabolism so that it can be modified for the improvement of agriculture. Although the enzymes of lignin biosynthesis have now been identified, we know relatively little about how their expression is regulated. Several relevant transcription factors have been isolated, but it is unclear how their expression and activity dictate or contribute to the allocation of photosynthate to lignin as opposed to other plant components such as cellulose, starch, or any other sinks for reduced carbon. We are in a unique position to explore how the amount of lignin in a plant is controlled because we have identified two novel plant-specific proteins (REF4 and RFR1) that are components of Mediator, a large multi-protein complex that facilitates interactions between DNA-bound transcription factors and RNA polymerase II to activate or repress the expression of downstream genes. Mutants of Arabidopsis that lack REF4 and RFR1 are viable and show little in the way of developmental changes, making them a tractable system in which to examine the function of Mediator. Of particular relevance to this project is that these mutants accumulate more phenylpropanoid end products including lignin. Plants carrying a mutant dominant form of REF4 show the opposite phenotype. Thus, REF4 and RFR1 appear to be components of a system that determines the amount of carbon allocated to the phenylpropanoid biosynthetic pathway. Considering that over 108 gigatons of lignin are synthesized annually in the biosphere, these proteins are important players in the global carbon cycle and represent important new opportunities for the manipulation of lignin synthesis in plants. Exploring novel metabolic pathways in Arabidopsis We have discovered a group of metabolites in Arabidopsis which we have named arabidopyrones (APs). APs are previously undiscovered molecules, the synthesis of which requires the activity of a ring-cleavage dioxygenase, a member of a class of enzymes of mostly unknown function that is conserved across the plant kingdom and beyond. By LC-MS and NMR we have shown APs to be substituted pyrones, the most abundant of which we have named arabidopyl alcohol. The structures of these molecules is highly reminiscent of compounds such as stizolobic and stizolobinic acids, as well as betalamic acid, a component of well-known pigments from beet, Portulaca and various cacti. These tyrosine-derived molecules are all 6-membered N- or O-containing heterocycles bearing substituted 2- or 3-carbon side chains. A common feature of the synthesis of these compounds is that they are derived by recyclization of extra-diol cleavage products of dihydroxyphenylalanine (DOPA). We have found that the only ring cleavage dioxygenase known to be encoded by the Arabidopsis genome (AtLigB) is required for arabidopyrone synthesis, presumably for cleavage of a dihydroxy-substituted precursor. The fact that ring cleavage dioxygenases have been conserved over 400 million years of plant evolution suggests that they are likely to play an important and conserved role in plant biochemistry. As a result, we believe that the activity of this class of proteins plays a more widespread and fundamental role in plant metabolism that remains to be discovered and that AtLigB has been recruited to serve a specialized role in AP biosynthesis in Arabidopsis. Dissection of AP synthesis in Arabidopsis using genetic, molecular and biochemical tools will shed light on the role(s) of this group of highly specialized catalysts in plants. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactchapple@purdue.edu  |
| Chen | Zhixiang | Plant Biology |
Zhixiang ChenAssociate Professor of Plant Pathology Current Research InterestsPlants are constantly exposed to microbial pathogens and through evolution have developed a battery of defense mechanisms for combating microbial diseases. The major research interest of our group is to decipher the regulatory network of plants defense responses. Transcriptional regulation of plant host genes is a central part of plant defense response and elucidating the complex regulatory mechanisms for the differential expression of plant genes holds the key to our understanding of the molecular basis of plant disease resistance. We are studying a family of plant transcription factors containing the novel WRKY zinc-finger DNA-binding motifs. WRKY transcription factors are found only in plants and are encoded by a large gene family with more than 70 members in Arabidopsis. A majority of the WRKY genes in Arabidopsis are rapidly induced upon pathogen infection, suggesting a major function of the gene family in plant defense responses. We are using both genetic and molecular approaches to understand the regulation and biological functions of plant WRKY genes in plant defense responses. In addition, we are studying posttranscriptional gene regulation and its roles in plant antiviral defense. Many eukaryotic organisms contain a group of novel RNA-dependent RNA polymerases (RdRPs) that synthesize small complementary RNAs (cRNAs) using cellular or viral RNA as templates. Certain members of RdRPs have recently been shown to be required for dsRNA-mediated gene silencing (RNA interference, RNAi). Recently we have identified a new plant RdRP that is induced by viral infection and defense-inducing compounds such as salicylic acid. Plants deficient in the inducible RdRP activity become more susceptible to viral pathogens. We are interested in understanding additional biological functions of the inducible RdRP and elucidating its action mechanisms in antiviral defense and RNAi. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactchen0@purdue.edu  |
| Chester | Julia | Integrative Neuroscience |
Julia ChesterAssociate Professor of Psychological Sciences Current Research InterestsThe main emphasis of Dr. Chester’s research program is to study the genetic, environmental, and neurobiological mechanisms that may promote or protect against the development of major mental diseases such as addiction, anxiety disorders, and schizophrenia. A primary area of her research is focused on examining the genetic and neurochemical mechanisms that regulate behavioral and motivational effects of alcohol. Dr. Chester has a strong interest in the role of stress and stress hormones in influencing alcohol-seeking behaviors and other behaviors that model abnormal psychological processes and psychiatric disease states in humans. Dr. Chester’s primary research focus is using genetic mouse models to explore how biological, genetic, and environmental factors, such as stress, may influence alcohol- and psychiatric disorder-related traits and how these factors interact with genetic propensity toward alcohol consumption. Her recent work has largely focused on developing an animal model of co-morbid alcohol use disorders and post-traumatic stress disorder (PTSD) and exploring target mechanisms for the treatment of these human disorders. Much of this work involves examining candidate genes and neurochemical and hormonal factors that may regulate brain-behavior relationships in these animal models. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactjcheste@purdue.edu  |
| Chmielewski | Jean | Chemical Biology, Membrane Biology, Microbiology, Immunology and Infectious Diseases |
Jean ChmielewskiProfessor — Organic and Bioorganic Chemistry Current Research InterestsAssembly and disassembly of peptides and proteins is a recurring theme in the research of our group. Self assembly is an essential element in the biological activity of biopolymers. In our research we design and synthesize self assembling peptide systems with novel binding and catalytic features. Our work spans many areas of interest including: · Covalent Stabilization of Large Helical Bundles · Peptide-Enhanced Liposomal Drug Delivery · Self-Replicating Peptides Work in these areas has the promise of producing viable ways of targeting drugs to the site of their action in the body, and has the potential to answer fundamental questions on the nature of the molecular origins of life. Many proteins also associate to form dimers and larger assemblies. The enzymes of HIV, for instance, rely on dimer formation for optimum catalytic activity. Transcription factors also form dimers, and this dimerization event is essential for their specific DNA binding properties. We have prepared unique inhibitors of dimerization in a number or areas including: · Enzymes of HIV: Protease and Integrase · Transcription Factors: E47, Fos/Jun, E2A-HLF, NF-kappaB · Restriction Endonucleases Inhibitors of this type may lead to potent classes of anti-AIDS or anti-cancer therapeutic agents, and also provide an increased understanding of the intermolecular contacts at protein subunit interfaces. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjchmiele@purdue.edu  |
| Chopra | Gaurav | Cancer Biology, Chemical Biology, Computational and Systems Biology, Integrative Neuroscience, Microbiology, Immunology and Infectious Diseases |
Gaurav ChopraAssistant Professor Current Research InterestsThe theme of my laboratory is Chemical Immunology. We are interested in understanding and chemically perturbing immune microenvironments in disease (cancer and neurodegeneration). We use immunological, synthetic, and proteome-scale methods to discover, design, synthesize and verify immunomodulatory chemical entities that are specific to immune cell subtypes (e.g. MDSCs, microglia, astrocytes, etc). We have developed a novel interactome based drug discovery and design platform that analyze compound-proteome interaction signatures at the genomic (proteomic) scale to determine drug behavior, in contrast to traditional single target approaches. We use machine learning to develop ‘computational assays’ to complement and guide chemical synthesis and biological experiments done in our laboratory and identify targets/networks to design drugs/combinations for specific cellular phenotype ex vivo and in vivo. My group and our collaborators have used this approach to identify putative drug leads (new synthetic leads, combination of existing drugs) using in vitro and in vivo preclinical studies for more than 10 different diseases including cancer, immunological, metabolic, infectious and genetic indications (castration resistant prostate, invasive bladder cancer, Alzheimer’s disease, type 1 diabetes, dental caries, dengue, herpes, drug resistant tuberculosis, etc). The two research themes of my lab in “data-driven chemical methodology” and “cell-specific chemical immunomodulation” will help us achieve our goal to develop immunomodulators (small molecules, cell conjugates, etc) that may be used therapeutically, as well as, “probes” to perturb immune microenvironments to explore cell-responsive pathways in different diseases. Students in our lab choose their research track based on their interest (see http://www.chopralab.com). We enjoy working with passionate individuals who care deeply about their research ranging from computational chemistry/biology to chemical biology wet-lab bench / animal work or both. Our lab culture is very open and encourages creativity and persistence to solve problems. Students in our lab will have the opportunity to identify problems they are most interested in solving. Our goal is to train students into well-rounded scientists so that they can develop an expertise in the lab. In summary, we are looking for smart, talented and passionate individuals so that we can learn from them as much as they learn from us. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactEmail: gchopra@purdue.edu  |
| Chubykin | Alex | Integrative Neuroscience |
Alex ChubykinAssistant Professor Current Research InterestsA major challenge in neuroscience is to understand how brain circuits process visual information and how these circuits are altered in Autism Spectrum Disorders (ASDs). To answer these questions, we use a multi-disciplinary approach combining optogenetics, electrophysiology, 2-photon imaging and behavior in genetic models of ASDs. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactchubykin@purdue.edu  |
| Clase | Kari | Biotechnology |
Kari ClaseAssociate Professor Current Research InterestsProf Clase’s lab investigates the mechanisms controlling neural cell proliferation and differentiation within brain tumors through proteomic and metabolic analysis. She is also interested in bringing grand challenge research problems into the classroom to engage students in the process of research and help them learn in an authentic interdisciplinary context. In order to facilitate this process, she explores the use of emerging technologies for learning and building collaborative communities. She currently teaches multiple courses covering topics in biotechnology, bioinformatics, biological design and drug discovery to engineers, scientists and technologists. Her currently funded projects include collaborators from multiple disciplines and an impact on students from K-12 to graduate education. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contact |
| Couture | John | Plant Biology |
John CoutureCurrent Research InterestsResearch in the Couture lab has four focal themes: 1) integrating chemical and landscape ecology to advance our understanding of the influence of trophic-level interactions on ecosystem functioning 2) examining the influence of environmental change on plant and insect ecology, across multiple levels of biological organization, to better understand how ecosystems will function in future environments 3) advancing the ability of hyperspectral data to characterize plant chemical and metabolic profiles 4) utilizing spectroscopy to improve precision agriculture Work in my lab combines analytical chemistry, hyperspectral and remote sensing technology, field-based measurements, manipulative experiments, and statistical modeling to test basic ecological theory, ultimately providing a better understanding of ecosystem functioning. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcouture@purdue.edu  |
| Cramer | William | Bimolecular Structure and Biophysics, Membrane Biology |
William CramerHenry Koffler Distinguished Professor of Biological Sciences Current Research InterestsConcept for the pathway and mechanisms of import into E. coli of the cytotoxic E colicins are based on structures of the outer membrane vitamin B12 receptor (BtuB; panels A, B, side and top views; J. Mol. Biol. 364: 716-734, 2006) and OmpF translocator (see below, E; EMBO J., 27, 2171-2180, 2008) which the colicins parasitize for their import. A 2.75 Å structure of the complex of the receptor-binding domain of the endoribonucleolytic colicin E3 (panel C) showed the elongate 100 Å long colicin domain to be bound in an oblique mode, in which it can fish for a second (OmpF) outer membrane translocator (Nat Struct Biol, 10, 948-954, 2003 ). A very similar structure was obtained for a complex of the receptor-binding domain of colicin E2 and BtuB (panel D) [J. Biol. Chem., 282: 2171-2180]. Circular dichroism in the far UV has been used to characteracterize the "unfolded" secondary structure of the N- and C-terminal peptides of colicin E3 that interact with the outer membrane receptors, BtuB and OmpF (Biochemistry, 45, 10199-, 2006). III. Discrete Ion Channel Formation by Alpha-Synuclein Alpha-Synuclein, a 140 amino acid cytosolic protein (Fig. below), implicated in the pathogenesis of Parkinson's Disease (PD), can exert its cellular function through interaction with membranes. This interaction has been studied mostly with oligomeric or aggregated "protofibrillar" forms of synuclein. In contrast to the view prevalent in the literature that the membrane-interactive form of alphaSynuclein is the oligomeric beta-stranded form that permeabilizes membranes, studies that we have carried out with J. - C. Rochet showed that monomeric synuclein in an alpha-helical conformation formed specific ion channels in planar bilayer membranes having a physiological lipid composition (Zakharov et al., Biochemistry, 2007). These channels, formed by insertion into the membrane bilayer, must result from the formation of a trans-membrane helical dimer or higher order oligomer. The synuclein trans-membrane channels could have a positive function in the metabolism of synaptic membranes through transport of biogenic amines. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactwaclab@purdue.edu  |
| Dadarlat | Maria | Computational and Systems Biology, Integrative Neuroscience |
Maria DadarlatDr. Maria Dadarlat
Current Research InterestsHumans make highly precise movements by combining information from various sensory modalities to plan and execute motor commands, a process called sensorimotor integration that must be learned through experience. We do so by forming internal models, dynamic neural maps between sensory information and motor commands. Despite evidence of the existence of such models, we have a limited understanding of how internal models form. The Dadarlat lab studies learning in the sensorimotor system by exposing adult animals to a novel sensorimotor pairing: using electrical stimulation to encode artificial sensory feedback during a behavioral task and state-of-the-art neural recording and mesoscopic 2-photon imaging to record changes in neural coding across sensory, parietal, and motor cortex during learning. In addition to systems neuroscience, the Dadarlat lab focuses on the development of artificial sensory feedback for neural prostheses, and approaches to enhance adult neuroplasticity to promote recovery from neural injury and disease.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmdadarla@purdue.edu
 |
| Dai | Mingji | Chemical Biology |
Mingji DaiAssistant Professor Current Research InterestsOur research focus on innovating in both the strategy and methodology of chemical synthesis, and applying them to solve problems of biological and medical importance and ultimately impact human health. We work on both natural and unnatural molecules with particular potential for the treatment of cancer, CNS disorders and infectious diseases. We view the completion of a synthesis as the beginning of a larger and deeper scholarly inquiry. It would enable us to profile the biology of the selected natural products and rationally designed analogs, decipher their mechanism of actions/cellular targets, and optimize the lead compounds into useful chemical probe and novel therapeutics development. We also work on creating unconventional and diverse small molecule libraries with the aim to target challenging but important disease targets, such as protein-protein interactions and transcription factors. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactdai41@purdue.edu  |
| Dangoudoubiyam | Sriveny | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Sriveny DangoudoubiyamAssistant Professor Current Research InterestsZoonotic ascarids and larva migrans Anthelmintic resistance in parasites host-pathogenic interaction of tissue-cyst producing coccidiaActive Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsdangoud@purdue.edu  |
| Das | Chittaranjan | Biomolecular Structure and Biophysics, Chemical Biology |
Chittaranjan DasAssistant Professor Chemistry Current Research InterestsCurrently, we are investigating the normal function of the neuronal DUB ubiquitin C-terminal hydrolase L1 (UCHL1)- a PD-associated, neuron-specific protein of unknown physiological function. Our efforts in this direction are aimed at developing cell-permeable small molecule inhibitors of UCHL1 that can be used to probe its function (both normal and disease-associated), determining its binding partners by affinity based purification from whole-cell extracts, and defining the molecular basis of how a naturally occurring variant of this enzyme- in which Ser at the position 18 is substituted by Tyr (called the S18Y polymorph)- provides protection from Parkinson's disease (PD). In addition to UCHL1, we are also conducting structural and mechanistic investigations of other related enzymes thought to be involved in fundamental biochemical processes such as DNA repair, histone modification, and endocytosis of plasma membrane proteins. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactcdas@purdue.edu  |
| Davisson | Vincent | Biomolecular Structure and Biophysics, Chemical Biology, Computational and Systems Biology |
Vincent DavissonProfessor of Medicinal Chemistry and Molecular Pharmacology Current Research InterestsOur primary interests are at the intersection of chemical and systems biology to enhance the drug discovery and development process. The research group uses both hypothesis-driven and technology-focused discovery approaches to address biomedical problems of relevance to cancers and neurodegenerative diseases. We engage a number of collaborative efforts to enhance the overall approaches to addressing these objectives. Our active core research program has two overall aims: a) To discover and develop selective antagonists/agonists of protein assemblies. The current target systems under investigation are involved in a variety of cellular roles including DNA replication-repair, cellular vesicle transport and pH control, and viral mediated oncogenesis. The specific target systems currently under investigation include cell proliferating nuclear antigen (PCNA), the vacuolar-ATPase (v-ATPase), and the human papillomavirus virus E6 protein (HPV-E6); all are implicated in different diseases. While our earlier research focused primarily on enzymes, all of the molecular systems under current investigation are considered non-classical or “undruggable” targets. Our efforts aim to discover and develop small molecule probes of these target systems to address their specific roles in disease contexts and serve as leads for drug discovery. A significant effort is devoted to exploring new approaches to design and discover useful chemotypes and drug leads for each of these target systems. In the process, we develop probes to test hypotheses regarding protein network interactions and define new target binding sites. Currently, biomolecular screening methods are being integrated with computational approaches and novel synthetic chemical libraries to enhance the successes of the discovery process. A long standing interest has been to further develop understanding of molecular mechanisms of drug actions. Part of the inspiration comes from the rich biological activities of natural products and their synthetic analogs. These molecular tools continue to provide rich sources for drug target discovery and/or serve as candidates for new therapeutics. We continue to pursue biochemical/proteomic and biophysical/structural biology approaches to understand and exploit the cellular pharmacology of natural products in future drug design. b) To develop novel high-content, quantitative, phenotypic cell-based screens for molecular discovery and evaluation which are predictive for modulation of cellular pathways and organelle functions. These collaborative efforts in platform development bridge chemical biology to bioengineering and computational sciences. The variation of biological response to chemical effects as a function of genetic content in a biological system is a problem for integration of high content systems. Using an integrated approach of genomics, proteomics, with flow and imaging cytometry, our collaborative efforts incorporate genetic variations in disease models into cell-based screening platforms. Our efforts integrate chemical, biochemical tools with automated cytometry, spectral imaging and bioinformatics to provide innovative biological screens for pharmacodynamic-monitoring. Insights from these efforts offer understanding of how best to target susceptibilities and stage drug therapies from discovery through development. The integration of these molecular analysis tools offer new technological interfaces that can also be applied toward clinical samples. These approaches using cell-based diagnostics when combined with molecular analysis are leading to the advancement of molecule cytomic platforms that can enhance the translation of discoveries into translational research and clinical practice. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactdavisson@purdue.edu  |
| Deng | Meng | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Meng DengAssociate Professor of Biological Engineering Current Research InterestsOur research lies at the interface of materials science, micro/nano-scale engineering, and cell biology/medicine. Of particular interest is to develop an integrated research program for both the fundamental understanding of cellular processes in tissue development and engineering effective biomaterial systems for tissue repair and regeneration. Our strategies embrace the mechanistic elucidation of various chemical and topographical cues on cellular processes, and application of advanced biomaterials and matrix technologies at the micro- and nanoscale. For example, advances in polymer science have allowed for the design of biomaterials for a specific medical application, while nanotechnology has provided a robust toolbox for the fabrication of tissue-specific architectures. Our work spans from basic science to translational technology. Specifically, we focus on the three thrust areas: (1) cell engineering, (2) advanced biomaterials, and (3) regenerative engineering. In the area of cell engineering, we are interested in understanding of cellular processes and research effective methods to modulate cell function (e.g., via delivery of inducerons); In the area of advanced biomaterials, we focus on rational design of new polymers and composites by exploiting synthetic chemistry and study of cell-material interactions. In the arena of regenerative engineering, we seek to develop effective bioengineered systems with cell-instructive cues for regeneration of complex tissues and tissue interfaces. There are natural synergies among all the three research areas.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Deng | Qing | Microbiology, Immunology and Infectious Diseases |
Qing DengAssociate Professor Current Research InterestsOur lab is interested in the mechanisms that regulate neutrophil migration and activation, more specifically their response to tissue injury and infection. The neutrophil is a hallmark of inflammation and neutrophilic inflammation, if not properly resolved, is the driving force behind a spectrum of human inflammatory diseases. Hopefully, our understanding of neutrophilic inflammation will help alleviate diseases such as arthritis, cancer and auto-inflammation. We are also interested in understanding pathogen-host interactions using zebrafish model and the strength relies on the combination of the genetics in both systems. We want to create a lab where happy scientists do solid science.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdeng67@purdue.edu  |
| Dilkes | Brian | Plant Biology |
Brian DilkesProfessor, Department of Biochemistry Current Research InterestsGenetics Evolution Hormones Metabolism Environmental Adaptation Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactbdilkes@purdue.edu  |
| Dudareva | Natalia | Plant Biology |
Natalia DudarevaDistinguished Professor, Department of Biochemistry Current Research InterestsPlants have exploited the language of small chemicals for interacting with their environment more extensively than any other types of living organisms. An amazing diversity of volatile molecules released by plants play essential roles in their growth, development, reproduction, defense, and communication, and influence atmospheric chemistry and climate. Formation of these compounds relies on primary metabolic networks for the supply of precursors for their biosynthesis. Research in my laboratory focuses on understanding of biochemical and molecular mechanisms controlling the formation of primary and secondary (phenylpropanoid and terpenoid) metabolites in plants using the power of genetic and biochemical approaches combined with metabolic flux analysis and modeling. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdudareva@purdue.edu  |
| Dykhuizen | Emily | Cancer Biology, Chemical Biology |
Emily DykhuizenAssistant Professor in Medicinal Chemistry and Molecular Pharmacology Current Research InterestsThe Dykhuizen lab is interested in using a combination of chemical and biochemical techniques to uncover the role of chromatin structure in tumor suppression. Uncovering the mechanisms of these complexes will reveal potential therapeutic avenues for cancers that currently have few therapeutic options. Three areas of research we are currently pursuing include (1) structural and biochemical analysis of chromatin modifying complexes, (2) genome-wide analysis to define the functional contribution of chromatin binding domains, such as bromodomains, PHD domains and chromodomains, within larger chromatin-modifying complexes, (2) identification an development of inhibitors of chromatin binding domains. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactedykhui@purdue.edu  |
| Ekenstedt | Kari | Computational and Systems Biology, Integrative Neuroscience |
Kari EkenstedtAssistant Professor
Current Research InterestsMy research group uses the latest molecular genetics and genomics tools to study Mendelian and complex genetic traits and diseases in canine populations. Our goals are to improve canine health through the understanding of genetic disease, allowing veterinarians to better predict, diagnose, and treat these syndromes, and to improve human health through the use of the dog as a biomedical model. We are working on over a dozen different naturally-occurring diseases in dogs, from musculoskeletal and neurologic to ophthalmic and even coat color. Specifically, we are currently investigating ectrodactyly, spinal abnormalities, dwarfism, ocular melanosis, progressive retinal atrophy, hypomyelination, "hidden" coat color alleles, and others. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkje0003@purdue.edu
 |
| Enders | Laramy | Microbiology, Immunology and Infectious Diseases |
Laramy EndersAssistant Professor, Department of Entomology Current Research InterestsInsects engage in diverse associations with microbial partners that range from parasitism to mutualism. Within both natural and agro-ecosystems the implications of microbial partnerships are important for determining how insects respond to environmental stress, overcome host plant defenses and spread diseases. For example, many insects are known for their symbiotic relationships with bacteria that play essential nutritional and defensive roles. In addition, insects are highly effective vectors of many plant pathogens. My research group is interested in understanding multi-trophic interactions between aphids, their symbionts and host plants. Ongoing research aims to investigate the extent to which the aphid microbiome mediates interactions with host plants and influences the transmission of plant pathogens. We primarily focus on a suite of aphid species that feed on toxic milkweed plants and several pest species that vector Barley yellow dwarf virus in cereal agro-ecosystems. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactlenders@purdue.edu
 |
| Engelberth | Abigail | Biotechnology |
Abigail EngelberthAssistant Professor Current Research InterestsAbigail Engelberth is an Assistant Professor in Agricultural and Biological Engineering and in Environmental and Ecological Engineering at Purdue University. She earned a B.S. and M.E. in Chemical Engineering from Iowa State University and a Ph.D. in Chemical Engineering from the University of Arkansas. Dr. Engelberth was a postdoctoral research assistant at the University of Maine and built a process model to emulate the conversion of hemicellulose into liquid fuels via anaerobic digestion. Dr. Engelberth’s research can be broken down into three sections. The first section is to identify new bioproducts or identify new sources from which to obtain existing bioproducts. The second piece is to develop methods to recover bioproducts. This piece will emply separations techniques to extract and/or purify the desired product. The third section involves a simulation of the process model for the recovery and purification steps in order to effectively scale-up the process. The process model will aid in the determination of how much it will 1) cost to produce the product, both in energy and operations, and 2) the market price of the product to either break even or to make a profit. The process mode adds a key feature – determining the actual value of the additional product – that is currently missing in many biomass co-product studies. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactaengelbe@purdue.edu  |
| Evans | Janice | Membrane Biology |
Janice EvansProfessor and Department Head of Biological Sciences Current Research InterestsThe Evans lab studies mammalian gamete biology, with a focus on how oocytes (egg cells) progress through meiosis and fertilization. This research has relevance not only to fundamental concepts in cell biology, but to the biology underlying reproductive success and failures, and the connections between fertility to overall health. Ongoing projects in the lab are addressing cell cycle regulatory processes at work during oocyte meiosis, and the functions of the cytoskeleton in various aspects of oocyte biology.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact765-494-4407  |
| Figueiredo | Marxa | Biotechnology, Cancer Biology, Microbiology, Immunology and Infectious Diseaes |
Marxa FigueiredoAssociate Professor Current Research InterestsImmunotherapy • bone metastatic tumors • repair of bone in tumor and arthritis models • mesenchymal stem cells Our laboratory develops strategies to leverage the immune system and promote repair of bone while controlling inflammation or tumor cell viability. The overall therapy goals are to (a) treat tumors and repair bone in tumor models and (b) treat and repair cartilage/bone in arthritis models. We have several ongoing projects to achieve these goals, including: 1. Immunotherapies such as C-term targeted cytokine IL-27 to control tumor inflammation (COVID19 related inflammation is a recent interest), for which we use gene delivery as a tool; 2. Targeting of receptors such as the laminin receptor using small molecules discovered in our laboratory, and 3. Understanding the biology of mesenchymal stem cells and how to utilize them as therapies. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmlfiguei@purdue.edu  |
| Flaherty | Daniel | Chemical Biology, Microbiology and Infectious Diseases |
Daniel FlahertyAssistant Professor Current Research InterestsResistance to commonly prescribed antibiotics is becoming an increasingly dangerous threat to society. Several strains of pathogenic bacteria have displayed resistance toward drugs that are commonly used as a last line of defense for treatment of these infections. Therefore, there is an urgent need to not only expand upon currently known antimicrobial chemical space but to also validate novel therapeutic targets for treatment of these infections. To this end the Flaherty lab utilizes a combination of traditional high-throughput screening techniques and contemporary fragment-based drug discovery practices to identify and optimize new chemical scaffolds for inhibition of novel antimicrobial targets. These techniques are combined with thermodynamic characterization of the ligand-protein binding event to gain a more intimate knowledge of the processes involved in the binding event. We then utilize this information to optimize inhibitors not only for increased potency but also for increased selectivity while maintaining optimal physicochemical properties of the molecule. The lab then uses these novel inhibitors to validate these enzymes as viable antibiotic therapeutic targets. One area of research that our lab is interested in is developing potent and selective inhibitors versus bacterial RNA degradation targets. RNA degradation is an essential cellular process for viability in all organisms. Bacterial RNA degradation pathways differ significantly from human pathways making targeting RNA degradation an attractive antimicrobial strategy. RNase E is highly conserved across all Gram-negative pathogenic species and catalyzes two essential steps in the mRNA degradation/tRNA maturation pathway. Furthermore, research has shown that knockout of this enzyme can lead to inhibition of two essential cellular processes resulting in a lower potential for resistance. However, there are no reported inhibitors for RNase E. Therefore, the Flaherty lab has embarked in a multidisciplinary effort to identify and optimize RNase E inhibitors utilizing a bi-lateral traditional HTS and FBDD approach coupled with structure-based drug design to arrive at novel and potent inhibitors. Furthermore, the use of thermodynamics to dissect the attributes that correlate with compound affinity provides a clearer picture of what drives binding. Finally, ligand-bound structural determination provides key information for structure-based design of inhibitors. Such inhibitors will be instrumental for in vivo validation of RNase E as a viable, broad-spectrum Gram-negative therapeutic target. While RNase E is essential for RNA degradation/maturation in all Gram-negative species, the enzyme RnpA is responsible for these processes in Gram-positive pathogens. RnpA is a small protein that has been shown to be an integral part of at least two RNA processing enzymatic complexes. Recent research has shown that inhibition of RnpA has a significant effect on both mRNA degradation and tRNA maturation. RnpA is also conserved across several Gram-positive species and represents a promising Gram-positive antimicrobial target. Utilizing the same strategy we have identified novel S. aureus RnpA inhibitors. Currently our laborotary is utilizing structural information and computational models to map where these inhibitors bind on the protein. Furthermore, we are working to elucidate the role different binding sites on RnpA play in each essential process. These inhibitors will prove essential for validating RnpA as a viable Gram-positive therapeutic target. A third project the Flaherty lab is currently pursuing is the development of potent and selective inhibitors for the human deubiquitinase UCHL1. The UCH family of enzymes has been implicated to play key roles in cancers of various tissues. Recently UCHL1 was even shown to be integral in deubiquitinating hypoxia-inducible factor 1a leading to metastasis. Currently there are two small molecule inhibitors for UCHL1, however these inhibitors significantly lack potency and selectivity versus other UCHs such as UCHL3 and UCHL5. Therefore, there is urgent need to develop a potent and selective inhibitor that can serve as a valuable tool to further elucidate UCHL1’s role in cancer biology and metastasis and validate UCHL1 inhibition as a viable anticancer strategy. Our lab collaborates with the laboratory of Dr. Chittaranjan Das in the Department of Chemistry at Purdue University to identify and develop best-in-class small molecule inhibitors versus UCHL1. The Das lab has solved the UCHL1 crystal structure and is a leader in UCHL1 biochemistry; thus, this collaboration provides an exciting opportunity to be at the forefront of the UCHL1 field. For more information please visit the laboratories webpage: http://www.mcmp.purdue.edu/faculty/?uid=dflaher Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdflaher@purdue.edu  |
| Fortin | Jessica | Chemical Biology, Integrative Neuroscience |
Jessica FortinAssistant Professor Current Research InterestsThe Fortin Drug Discovery Laboratory is working to discover new treatments for Alzheimer’s disease, Parkinson's disease, and type 2 diabetes. We design and evaluate the impact of small molecules on the conformational changes of prone-to-aggregate proteins. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactfortinj@purdue.edu  |
| Fox | Edward | Integrative Neuroscience |
Edward FoxAssociate Professor of Psychological Sciences Current Research InterestsHow do we make decisions about what we eat - how much we eat, when we eat, or how often we eat? One neural system important in making these decisions is the component of the autonomic nervous system carried by the vagus nerve. Currently, a major focus for us is the sensory component of the vagus nerve because it is one of the most significant inputs for regulating metabolism and food intake. Traditional methods have not provided a means for independently manipulating the numerous vagal sensory pathways to determine their functions. Thus, we are taking advantage of mouse genetics and transgenic technology in combination with nerve mapping, immunohistochemical methods, and sophisticated behavioral methods to dissect the functions of these pathways Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactfoxe@purdue.edu  |
| Francis | Gregory | Integrative Neuroscience |
Gregory FrancisProfessor of Psychological Sciences Current Research InterestsMy laboratory focuses on three main topics. First, I study neural network models of human behavior. I have studied the dynamic characteristics of a neural network model of visual perception and, through computer simulation, demonstrated that the model's behavior matches human data on dynamic vision. The model properties are now being further examined to explain additional human data. Additional research investigates the dynamic properties of recurrent networks for pattern recognition and memory. I've made available an on-line set of simulations for models of backward masking. These simulations allow anyone to use the models without having to write their own code. Second, I explore human-computer interactions. Computer information is often presented in a menu format where a user moves through a sequence of menus to reach desired information (think of an ATM money machine). My research has identified a quantitative method for organizing these types of menus so that they are easy to use. Currently, these methods are organized in MFDTool, a software aid for the design of multifunction displays. Much of this research has been supported by the U.S. Army Aeromedical Research Laboratory at Ft. Rucker, AL. Finally, I am involved in the creation of a novel set of teaching tools. The Cogntive Psychology Online Laboratory provides a set of java program that allow a user to explore some online demonstrations of experiments in cognitive psychology. A similar project called the Visual Perception Online Laboratory is dedicated to experiments related to vision. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactgfrancis@purdue.edu  |
| Freeman | Jennifer | Cancer Biology, Computational and Systems Biology, Integrative Neuroscience |
Jennifer FreemanProfessor of Toxicology Current Research InterestsDr. Freeman's research efforts are focused on defining the underlying genetic and epigenetic mechanisms of toxicity of environmental stressors with current emphasis on pesticides, metals, PFAS, radiation, and emerging contaminants. Projects are defining the immediate adverse impacts of a developmental exposure, the lasting adverse impacts of this developmental exposure throughout the lifespan, and the analysis of subsequent generations linking genetic, epigenetic, and phenotypic assessments. These studies are investigating the developmental origin of health and disease pathogenesis with a specific focus on neurological disorders and diseases, neuroendocrine dysfunction, and cancer with a goal of understanding the role of exposure to the environmental stressors in these adverse health outcomes. In addition, projects are investigating the role of structural genetic variation in toxicity responses. All projects are currently utilizing the zebrafish vertebrate model system as a tool to investigate toxicity. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjfreema@purdue.edu  |
| Gelvin | Stanton | Microbiology, Immunology and Infectious Diseases, Plant Biology |
Stanton GelvinProfessor Current Research InterestsCrown gall is a neoplastic disease caused by the infection of dicotyledonous plants by virulent stains of the Gram-negative soil bacterium Agrobacterium tumefaciens. During the process of infection part of a bacterial plasmid, called the tumor inducing (Ti) plasmid, is transferred from the bacterium to the plant, where it stably integrates into the nuclear DNA. This transferred, or T-DNA, can be expressed as mRNAs which are translated. Tumorous lesions result, as well as the production of rare compounds called opines which the bacterium can utilize as an energy source. In our laboratory we have been interested in the molecular mechanism of Ti-plasmid transfer, integration, and expression. To define these mechanisms, we are investigating both Agrobacterium and plant genes required for transformation. Among the many approaches we are taking is the identification of Arabidopsis mutants that are resistant to Agrobacterium transformation (rat mutants) and Arabidopsis mutants that are hyper-susceptible to Agrobacterium transformation (hat mutants). We have identified more than 125 rat mutants and 10 hat mutants are analyzing the functions of the mutated genes in the transformation process. Many of these mutations are in genes involved in chromatin function, nuclear targeting, cytoplasmic trafficking via the actin cytoskeleton, and cell wall biosynthesis. In the course of characterizing plant proteins involved in transformation, we have developed a novel system to image the interaction of Agrobacterium virulence proteins with plant proteins. This system, bimolecular fluorescence complementation, allows one to visualize protein-protein interactions in living plant cells. Finally, we have used microarray and bioinformatic analyses to identify plant genes that respond to Agrobacterium infection. Many of these genes are involved in host defense responses. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactgelvin@purdue.edu  |
| Ghosh | Arun | Chemical Biology |
Arun GhoshProfessor of Chemistry and Professor of Medicinal Chemistry Current Research InterestsMy research group is involved in multidisciplinary research projects in the areas of synthetic organic, bioorganic and medicinal chemistry. Of particular interest, we are investigating: - Synthesis and biological studies of Bioactive Natural Products - Design and synthesis of Molecular Probes for Bioactive Peptides and Proteins - Structure-based Design of Enzyme Inhibitors for Alzheimer's Disease and AIDS - Development of Asymmetric methodologies (Catalytic and Stoichiometric) - Multicomponent Reactions (MCR) for Highly Functionalized Products The total synthesis and exploration biology of various medicinally important natural products are important parts of my group's research. These interests include the development of new synthetic methodologies, general strategies for synthesis and the study of important structure-function relationships. In this context, we are investigating the chemistry and biology of anticancer agents laulimalide, amphidinolides, peloruside A and opioid receptor antagonist salvinorin D. Another important research area in my group is the design and synthesis of molecular probes and nonpeptidal turn-mimics for biologically active peptides and proteins. We are currently studying critical ligand-binding site interactions of various proteolytic enzymes. This includes, memapsin 2, a very significant target for Alzheimer's disease as well as, HIV protease whose clinical effectiveness for the treatment of AIDS has been well recognized. Furthermore, we are involved in the development of asymmetric reactions based upon various intermolecular or intramolecular metal chelations. Of particular interest, we are investigating a variety of asymmetric syntheses including syn- and anti- selective aldol, hetero Diels-Alder, conjugate addition and multicomponent (MCR) reactions. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactakghosh@purdue.edu  |
| Gimble | Frederick | Biomolecular Structure and Biophysics |
Frederick GimbleAssociate Professor of Biochemistry Current Research InterestsThe sequence of the human genome will help identify genetic mutations that cause disease, and a central goal in the post-genomic era will be to develop tools to correct these errors. The repair of complex genomes requires having reagents available that 1) are capable of locating a specific sequence from among several hundred megabases of non-specific DNA and 2) are able to catalyze specific molecular modifications of the DNA that either initiate an endogenous repair pathway or effect repair directly. Our laboratory has focused on designing and engineering homing endonucleases with novel functions that have the potential to facilitate DNA repair and other molecular processes. Homing endonucleases are encoded by mobile DNA elements that propagate between individuals within a population by “homing,” and between species through lateral transmission. These enzymes initiate homing by introducing a double-strand break at a single genomic target sequence situated within a cognate allele that lacks the mobile element. A long term goal of our group is to harness the extreme DNA sequence specificity of homing endonucleases to catalyze specified events at targeted genomic loci. Our laboratory applies directed evolution and rational design strategies that are based on structural and phylogenetic information in order to design homing endonucleases with novel functions. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactfgimble@purdue.edu  |
| Giordano | Nicholas | Integrative Neuroscience |
Nicholas GiordanoProfessor of Physics and Assistant Dean of Science Current Research Interests1)The physics of nanostructures and mesoscopic systems My group studies the properties of very small metallic systems, including such phenomena as the Kondo effect in one and two dimensions, the behavior of domain walls in very narrow ferromagnetic wires, and fluid flow in extremely small structures. Students who have recently been involved in this work include Todd Jacobs, Baris Cetin, Jiangtao Cheng, and Jacob Millspaw. More details along with some of our recent papers are given on our mesoscopic page. 2) Musical acoustics and the physics of the piano We are studying why the piano sounds like a piano. We are developing a physical model of the piano - this model will use Newton's laws to calculate the motion of all of the pieces of a piano along with the sound pressure which is produced. This work also involves experimental studies of piano hammers, strings, and soundboards. This work has been done by Andy Korty, James Winans, Stu Dietz, John Millis, James Roberts, and Laura Rueff. More details of this work, including some of our recent publications are given at our piano www pages. 3) Computational neuroscience We are starting several projects in the area of computational neuroscience. This work has to do with the nervous system, and how it does various sorts of "computations". We are interested in the detailed physics, biophysics, and neuroscience of how signals are generated and propagate in the brain, and in how the brain processes these signals. These projects are described in more detail on our neuroscience page, where our publications in this area can be found. This work has been done by Jasper Wang and Zhouhan Liang. 4) Computational physics I have a long standing interest in doing and teaching computational physics. This work includes the musical acoustics described above, along with my book Computational Physics (Prentice-Hall) which was developed when I taught a course on this topic. 5) Guitar acoustics We have just begun some modeling studies of the guitar. Some information on this work and some calculated guitar tones can be found here.
PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactgiordano@purdue.edu  |
| Georgen | Craig | Biotechnology |
Craig GeorgenAssociate Professor Current Research InterestsWe focus on developing advanced imaging techniques to study both cardiac and vascular disease. Since cardiovascular disease is the leading cause of death and disability in the world, fundamental understanding of heart and vascular disease initiation and progression is necessary to develop the next generation of therapeutics and devices. Non-invasive imaging has become vital for the detection and monitoring of disease progression, aiding in the development of technologies that will positively impact the clinical care of patients. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactcgoergen@purdue.edu  |
| Golden | Barbara | Biomolecular Structure and Biophysics, Chemical Biology, Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Barbara GoldenAssociate Professor of Biochemistry Current Research InterestsThe focus of our research is the structure and folding of catalytic RNAs. Unlike proteins, RNAs have a highly charged backbone, only 4 different monomeric units (compared to the 20 amino acids that make up proteins) and functional groups that are largely sequestered within the majorand minor grooves of the double helix. Yet, in the presence of magnesium ion, many RNA molecules have stable, globular, tertiary structures that support biological catalysis. To understand how these molecules fold and function, we are investigating RNA structures by X-ray crystallography. Our research is concentrated on RNA enzymes, or ribozymes, that display catalytic activity in the complete absence of protein cofactors. Group I introns catalyze transesterification reactions by activating a guanosine nucleophile for attack on the phosphodiester backbone. The structure of an active ribozyme derived from the catalytic core of the Tetrahymena LSU group I intron was recently solved at 5 Å resolution. This structure reveals extensive interactions between two domains that construct an active site composed of RNA. These studies will be extended to obtain a higher resolution picture of a group I intron and examine the intron's interaction with its substrates. A second RNA enzyme, RNase P also catalyzes phosphodiester cleavage. In contrast to the Tetrahymena ribozyme, RNase P uses water as the nucleophile. The other substrate for the reaction is a precursor tRNA, cleaved by the enzyme after the 5' leader sequence to generate a mature tRNA. RNase P from Bacillus subtilis consists of a 14 kD protein and the 405 nucleotide P RNA. We are using X-ray crystallography to investigate the active site of this enzyme. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactbarbgolden@purdue.edu  |
| Gowher | Humaira | Biomolecular Structure and Biophysics, Cancer Biology |
Humaira GowherAssociate Professor, Department of Biochemistry Current Research InterestsThe overarching goal of our research is to elucidate epigenetic mechanisms that control cell identity and determine how these mechanisms are disrupted in cancer. The research specifically focuses on the activity of distal regulatory elements, called enhancers, and insulators of developmental genes by using mouse embryonic stem cells and embryonal carcinoma cells as model systems. In addition, we also study biochemical mechanism/s of DNA methyltransferases and the effect of mutations commonly found in cancer and other developmental disorders. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthgowher@purdue.edu
 |
| Gribskov | Michael | Biomolecular Structure and Biophysics, Cancer Biology , Chemical Biology, Computational and Systems Biology |
Michael GribskovProfessor of Biological Sciences and Computer Science Current Research InterestsGenomic and transcriptomic analysis of model and non-model organisms, the application of pattern recognition and machine learning techniques to biomolecules, the design and implementation of biological databases to support molecular and systems biology, development of methods to study RNA structural patterns, and systems biology studies of cancer and human disease. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactmgribsko@purdue.edu  |
| Hall | Mark | Cancer Biology |
Mark HallAssociate Professor of Biochemistry Current Research InterestsResearch in the Hall lab currently focuses on the regulation of mitosis by post-translational processes, including dynamic phosphorylation and ubiqutin-mediated protein degradation. One current project is studying mechanisms of anaphase-promoting complex function. The anaphase-promoting complex is a large E3 ubiquitin ligase that controls the metaphase-anaphase transition, mitotic exit, and G1 phases of the cell cycle among other things. It is the indirect target of existing cancer chemotherapies like taxol and an attractive target for development of new chemotherapies. Our primary interest is understanding how this enzyme is regulated and how it recognizes the correct substrates at the right time to promote their degradation. A second project focuses on characterizing the phosphatases responsible for completing mitosis and cytokinesis during the eukaryotic cell cycle. Much work has focused on understanding the kinases that control cell division but much less is known about their counteracting phosphatases. We are interested in understanding how multiple phosphatases with distinct specificities act together to coordinate mitotic exit and cytokinesis, and maintain genome stability. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmchall@purdue.edu  |
| Handa | Avtar | Plant Biology |
Avtar HandaProfessor of Molecular Genetics Current Research InterestsSignificant decrease in crop productivity results from high temperature stress and inability to control senescence processes during postharvest storage of fruit and vegetable crops. The Goal of research is to characterize the molecular mechanisms, including identifying genes, which regulate biological processes in crops that effect agronomical performance, postharvest quality and shelf life and yield of processed products made from fruit crops. Among the projects being perused include: 1) Signal transduction pathways by which polyamines regulates various plant growth and developmental processes including levels of antioxidants, minerals and vitamins in plant tissues; 2) Functions of protein chaperon in regulating stress responses, especially to heat stress, during plant growth and development effecting crop yield and biomass accumulation; and 3) Biochemical mechanisms that regulate fruit shelf-life, texture, ripening and processing quality. Approach: Transgenic genetics, Biochemistry and physiology, Molecular biology, Cell biology PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactahanda@purdue.edu  |
| Hanna | Jason | Cancer Biology |
Jason HannaJason Hanna, PhD
Current Research InterestsMy lab studies microRNAs and angiosarcomas which are aggressive vascular sarcomas from blood and lymphatic endothelial cells. Despite the extremely poor prognosis for patients, the genetic drivers and molecular mechanisms of these tumors remain unclear. In recent work, we found that Dicer1 and microRNAs may function as critical tumor suppressors. We have gone on to generate additional tumor models investigating other genes known to be altered in patients. We leverage these genetic models to study tumor initiation, progression, and metastasis in vivo and in cell line models. We aim to 1) discover the genetic drivers and critical microRNAs involved in angiosarcoma development, 2) identify the key mediators of angiosarcoma metastasis, and 3) design and evaluate precision therapeutics to benefit patients of this devastating and understudied disease.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthannaja@purdue.edu
 |
| Hazbun | Tony | Cancer Biology, Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Tony HazbunAssociate Professor Current Research InterestsThe research philosophy of the Hazbun Lab is to approach biological questions from a network perspective and also utilize traditional scientific reductionist approaches to dissect and understand biological phenomena and mechanisms. Our research program uses systemwide approaches in yeast to investigate biological pathways involved in mitosis and cellular homeostasis related to human disease. We also use chemogenomic profiling to identify antifungal targets and characterize fungal gut metabolomics. In addition to these approaches we also use conventional biochemical, genetic and biophysical approaches to validate and interrogate the cellular network nodes and connections. The lab currently has three major areas of research:
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactthazbun@purdue.edu  |
| Heinz | Michael | Integrative Neuroscience |
Michael HeinzAssistant Professor, Departments of Speech, Language, and Hearing Sciences and Biomedical Engineering Current Research InterestsMy research focuses on understanding and quantifying relations between physiological and perceptual effects of sensorineural hearing loss (SNHL). In quantifying these relations, I have a long history of NIH funding and published work involving the coordinated use of neurophysiology, psychoacoustics, and computational modeling. My doctoral training in Speech and Hearing Science at MIT involved the theoretical and computational advancement of classic approaches to relating physiological responses to psychophysical performance, with the motivation to incorporate physiological properties associated with SNHL. My post-doctoral training in Biomedical Engineering at Johns Hopkins University involved single-unit neurophysiological recording in animals with permanent noise-induced hearing loss. As a faculty member at Purdue, I direct the Auditory Neurophysiology and Modeling Laboratory, where we have been combining these neurophysiological and modeling approaches. Our projects have focused on characterizing numerous effects of permanent SNHL on temporal coding using single-unit recordings from auditory-nerve fibers. We have also compared numerous non-invasive physiological assays (e.g., OAEs, ABRs, EFRs) to the single-unit data following SNHL. We are also measuring behavioral consequences of noise-induced hearing loss in our lab using the same species and noise exposures used in our physiological studies. We have expanded our work to include both temporary and permanent hearing losses due to noise exposures, along with several ototoxic models of SNHL (e.g., carboplatin, gentamycin, and furosemide). Recently, we have been leveraging our in-depth knowledge of relationships between single-unit and non-invasive assays of auditory function in various chinchilla models of SNHL to develop physiological biomarkers for applications in precision audiology. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmheinz@purdue.edu  |
| Helie | Sebastien | Integrative Neuroscience |
Sebastien HelieAssistant Professor of Psychological Sciences Current Research InterestsResearch interests include computational cognitive neuroscience, cognitive neuroscience, categorization, automaticity, rule learning, sequence learning, skill acquisition, intuition in decision-making and creative problem solving. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactshelie@purdue.edu  |
| Henderson | Gregory | Computational and Systems Biology |
Gregory HendersonAssociate Professor Current Research InterestsLipid metabolism plays a critical role in the supply of fuel to various tissues, and it is physiologically important to alter the rate of lipid shuttling between tissues and cellular compartments as energy needs change throughout the day. Improper regulation of lipid metabolism leads to enhanced risk for metabolic and cardiovascular disease. The Henderson Lab studies lipid metabolism at a biochemical level and in vivo, with focus on lifestyle factors such as energy balance, exercise, and diet selection. Specifically, the research group studies responses of free fatty acid mobilization, lipoprotein kinetics, and accumulation of various lipids within tissues such as the liver. The overall goal is to understand how lipid metabolism responds to physiological stressors and healthful lifestyle factors. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactgchender@purdue.edu  |
| Hill | Catherine | Membrane Biology, Microbiology, Immunology and Infectious Diseases |
Catherine HillCatherine A. Hill, Ph.D. Current Research InterestsInfectious diseases transmitted by mosquitoes and ticks represent significant global threats to public health and biosecurity. We are entering an era in which our ability to control arthropod-borne diseases such as Zika, dengue, malaria and Lyme disease is threatened by increasingly widespread drug and insecticide resistance. The goal of our research program is to develop novel, environmentally benign strategies to control mosquito and tick vectors of disease. Our research combines genomic, computational, molecular and drug discovery approaches to understand the biology of arthropod vectors. Our basic research program investigates how arthropod vectors transmit pathogens and parasites to their vertebrate host. We seek to identify and characterize novel molecular targets for the development of new insecticides and transmission-blocking vaccines. Our aim is to understand the biology and signaling of arthropod G protein-coupled receptors (GPCRs) and advance these receptors as novel molecular targets for insecticide discovery. Our applied research program uses drug discovery approaches to develop new mode-of-action vector insecticides that are safer for humans and the environment. We work across two PULSe Training Groups – “Immunology and Infectious Diseases” and “Membrane Biology”. On campus research collaborators include Professors Richard Kuhn, Department of Biological Sciences and Val Watts, Medicinal Chemistry and Molecular Pharmacology. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthillca@purdue.edu  |
| Hockerman | Gregory | Integrative Neuroscience |
Gregory HockermanAssociate Professor of Med Chem Mol Pharm Current Research InterestsVoltage-gated calcium channels are key players in a large array of physiological processes including contraction of cardiac, vascular and skeletal muscle, release of neurotransmitters from nerve terminals, gene expression, and hormone secretion. The long-range goal of our studies is to contribute to the development of drugs that can modulate voltage-gated calcium channels in a tissue and type selective manner to treat cardiovascular disease, stroke, and type II diabetes. Recent work in my lab has centered on describing the binding site for the BZP diltiazem in the Cav1.2 L-type channel, as well as the Ca2+ binding site in the channel that modulates the affinity for diltiazem and verapamil. We are currently using chemical oxidizing reagents in conjunction with mutant channels to understand how ischemia my modulate channel activity. Our current research is focused on the role of Cav1.2 and 1.3 channels in insulin secreting cells. My lab has developed mutant versions of Cav1.2 and 1.3 that are resistant to the DHP class of L-type channel blockers (Cav1.2/DHPi and Cav1.3/DHPi), but remain sensitive to the BZP diltiazem. After introducing these mutant channels into insulin-secreting INS-1 cells, we are able to functionally isolate either Cav1.2 or Cav1.3 channels by "turning off" endogenous L-type channels with the DHP nifedipine. Using this approach, we found that Cav1.3, but not Cav1.2, channels are coupled to glucose-stimulated insulin secretion from INS-1 cells. Further, we found that Cav1.3, but not Cav1.2, channels are coupled to glucose-stimulated oscillations in intracellular Ca2+ concentrations. Finally, we found that insulin secretion stimulated by the incretin peptide GLP-1 is preferentially coupled to Cav1.3. Our lab is also collaborating with other groups to study the roles of Cav1.2 and 1.3 channels in cardiac myocytes and endocrine cells using the same strategy. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthockerma@purdue.edu  |
| HogenEsch | Harm | Microbiology, Immunology and Infectious Diseases |
Harm HogenEschAssociate Dean for Research PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contact765-496-3487  |
| Hrycyna | Christine | Biomolecular Structure and Biophysics, Cancer Biology, Chemical Biology, Membrane Biology, Molecular Evolutionary Genetics |
Christine HrycynaAssociate Professor of Chemistry Current Research InterestsOur main research interests are in the field of multidrug resistance in human cancer. Although numerous cancers can be treated successfully with surgery, radiotherapy or chemotherapy, many cancers are intrinsically resistant to anti-cancer drugs or become resistant through the course of treatment. This broad-based cellular resistance to anti-cancer drugs results, in large part, from expression of multidrug transporters. Many of these transporters are members of the ATP-binding cassette (ABC) superfamily and require ATP hydrolysis for function. We study two of these pumps, the newly discovered protein, MXR1 as well as P-glycoprotein. Since a variety of different human cancers express these proteins at levels sufficient to confer multidrug resistance during therapy, it is important to understand how these transporters function in order to combat this phenomenon clinically. Taking biochemical, molecular genetic, biophysical and cell biological approaches, we focus on elucidating the molecular mechanisms of substrate recognition and how the energy of ATP hydrolysis is transduced into drug transport. A complete understanding of these proteins could lead to the development of new therapeutic agents that could greatly facilitate the treatment of a large number of human cancers. We are also interested in the post-translational processing of eukaryotic proteins. Many proteins involved in signal transuction, such as the ras proteins, are modified at their C-termini, targeting them to the appropriate intracellular location where they function. These proteins are initially synthesized with a C-terminal -CaaX sequence (where C is cysteine, "a" is an aliphatic residue and "X" can be any number of amino acids) and undergo three serial reactions including isoprenylation of the cysteine, proteolysis of the three terminal amino acids (-aaX) and a-carboxyl methyl-esterification of the cysteine. Our lab is investigating the methyltransferase responsible for the final modification and the possible role reversible methylation plays in cellular trafficking and localization of -CaaX containing proteins. Interestingly, when oncogenic ras is not methylated, it is not properly localized and is no longer able to transform cells. Therefore, novel inhibitors of C-terminal isoprenylcysteine methylation may prove to be useful clinical anti-cancer agents. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthrycyna@purdue.edu  |
| Huang | Rong | Chemical Biology |
Rong HuangAssociate Professor Current Research InterestsOur lab applies a multidisciplinary approach to 1.) Elucidate the mechanisms and functions of epigenetic modifications including methylation and acetylation; 2.) Rational design and synthesize selective and potent inhibitors for methyltransferases and acetyltransferases; 3.) Develop novel therapeutic agents for cancer and other diseases through targeting epigenetic pathways. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthuang-r@purdue.edu  |
| Huber | Jessica | Integrative Neuroscience |
Jessica HuberProfessor, Department of Audiology and Speech Sciences Current Research InterestsJessica E. Huber, Ph.D., CCC-SLP, is a Professor of Speech, Language, and Hearing Sciences in the College of Health and Human Sciences at Purdue University. The aim of her National Institutes of Health funded research program is to develop a theoretical account of the multiple factors that influence speech production and cognitive change in older adults with and without Parkinson disease (PD) and to translate findings to clinical treatment resulting in improvements in communication. She is the inventor of a small wearable device, the SpeechVive device, to treat communication impairments in people with PD. The device elicits the Lombard effect that can be exploited to improve speech clarity in individuals with PD while bypassing cognitive and sensory impairments. Her current research continues to examine the therapeutic outcomes and physiologic effects of a number of speech therapy techniques including respiratory muscle strength training and the SpeechVive. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactjhuber@purdue.edu  |
| Hudmon | Andy | Integrative Neuroscience |
Andy HudmonAssociate Professor of Medicinal Chemistry and Molecular Pharmacology Current Research InterestsThe long-term goal of the Hudmon laboratory is to elucidate how protein kinases and other effectors function as specialized molecular machines and assemble with their substrates and regulators to form signaling modules in excitable cells like neurons and myocytes. The clinical relevance of these studies hinge upon the role that these signaling complexes play in aberrant calcium signaling; processes that contribute to neurodegeneration following excitotoxicity stimuli like brain trauma and hyperexcitability with chronic pain and lethal arrhythmias in the failing heart. My laboratory focuses on kinases, key signaling molecules downstream of multiple second messengers which represent a significant portion of the druggable effectors being targeted for therapeutic utility. Current research focuses on the Ca2+/calmodulin activated protein kinase (CaMKII); a serine/threonine kinase regulating diverse substrates in response to calcium signaling. While CaMKII is well known for its role as a key mediator of synaptic plasticity and learning/memory, its activity has also been implicated in most organ systems of the body where its substrates are linked to diverse processes. Current research is aimed at understanding how CaMKII contributes to arrhythmogenesis in heart failure through its regulation of voltage-gated sodium and potassium channels as well as understanding how CaMKII signaling contributes to neuronal degeneration and dysfunction during excitotoxicity. Current studies are underway to develop small molecule and highly specific peptides to therapeutically modulate CaMKII in these disease states.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactPhone: 765-496-6389
 |
| Hunt | Greg | Molecular Evolutionary Genetics |
Greg HuntAssociate Professor of Entomology Current Research InterestsWe are studying behavioral genomics in honey bees, focusing on defensive behavior and foraging behavior. The high recombination rate of the honey bee allows us to map genes that influence behavior to small regions of the chromosome. We are currently studying candidate genes for defensive behavior to determine which ones are responsible for the natural variation observed, and the mechanisms involved. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactghunt@purdue.edu  |
| Lyer-Pascuzzi | Anjali | Plant Biology |
Anjali Lyer-PascuzziAssistant Professor Current Research InterestsDr. Iyer-Pascuzzi’s research investigates the mechanisms that plant roots use to perceive and respond to the environment. There are two primary areas of research in the lab. The first is focused on understanding the molecular basis of plant resistance to bacterial wilt, caused by Ralstonia solanacearum. Ralstonia is a devastating soil-borne pathogen that first infects root systems. Despite the devastation it causes, little is known regarding the networks that underlie resistance or susceptibility, and root responses to R. solanacearum are unclear. Using both tomato and Arabidopsis, we focus on understanding resistance responses at three levels of root development: root cell types, root developmental stages, and root architecture. Current questions include, what are the spatio-temporal dynamics of pathogen invasion in resistant and susceptible genotypes? How are different root cell types and developmental stages affected by bacterial wilt? What are the gene regulatory networks involved in the response to bacterial wilt within each cell type? We use a combination of cell biology, genetics, and genomics approaches to address these questions. The major goal of this research is to identify novel forms of resistance to bacterial wilt. Our second area of research is centered around the role of Nodule Inception-Like Proteins (NLPs) in root development. NLP proteins are a unique family of transcription factors found in a wide diversity of plant species. We are studying the molecular mechanisms through which these proteins mediate root development and stress responses in Arabidopsis. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactaiyerpas@purdue.edu  |
| Jeong | Hyunyoung | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Hyunyoung JeongProfessor, Department of Industrial and Physical Pharmacy Current Research InterestsIntestines harbor trillions of microbes that have evolved in the milieu of a diverse diet-derived small molecules. Gut microbiome (the collection of genetic materials harbored by the gut microbes) contains thousands of distinct genes with an enormous capacity to catalyze chemical reactions. Their functions, however, remains largely unknown. Jeong and Lee lab has been investigating the gut microbiota as (1) a drug-metabolizing organ and (2) a modulator of host response to drugs. Based on the expertise of Dr Jeong (a pharmacologist) and Dr Lee (a microbiologist), we identify and characterize the microbial factors involved in drug metabolism as well as host-microbe interaction that leads to altered drug efficacy and toxicity.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyoungjeong@purdue.edu  |
| Jiang | Wen | Biomolecular Structure and Biophysics, Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Wen JiangProfessor
Current Research InterestsThe research projects in my group are focused on method development and applications of cryo-EM to various biological systems. Current application projects include 1) structural basis of assembly and infection mechanism of dsDNA tailed bacteriophages and human noroviruses; 2) structural studies of protein complexes involved in epigenetics, signal transduction, and protein degradation; 3) structural based discovery of antivirals and cancer drugs. We are also interested in developing methods to improve all aspects of cryo-EM techniques including image processing algorithms, high-performance computing, data collection automation, and sample preparation. Current projects include 1) virtual reality-augmented cryo-EM training system (CryoVR); 2) genetic, biochemical, and physical approaches to stabilize protein complexes and to reduce flexibility to allow high-resolution 3-D reconstruction; 3) computational algorithm and software to process cryo-EM 2-D images and to reconstruct 3-D structures at near-atomic resolutions (1.5-4 Angstrom) Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjiang12@purdue.edu  |
| Johnston | Cliff | Biomolecular Structure and Biophysics, Microbiology, Immunology and Infectious Diseases |
Cliff JohnstonProfessor of Agronomy Current Research InterestsResearch interests are focused on understanding the surface chemistry of soil and environmental particles in the broader context of biosphere science. Using optical, laser-based, and structural methods we study soil and environmental particles and the interaction of both natural and anthropogenic compounds with soil and environmental particles. Current research projects range from fundamental studies of clay minerals and hydrous oxides to earthworms, carbon sequestration and emission, environmental fate and transport of antibiotics, aryl hydrocarbon receptor ligands (e.g, dioxins) and pesticides in soil. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactclays@purdue.edu  |
| Jones-Hall | Yava | Microbiology, Immunology and Infectious Diseases |
Yava Jones-HallAssistant Professor Current Research InterestsInflammatory Bowel Disease (IBD): Using mouse models of IBD, my lab studies the interactions between the host immune system, specifically, Tumor Necrosis Factor and the colonic microbiome during the progression from acute to chronic colitis and colon cancer. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactyljones@purdue.edu  |
| Kasinski | Andrea | Cancer Biology |
Andrea KasinskiWilliam and Patty Miller Assistant Professor of Biological Sciences Current Research InterestsMicroRNAs (miRNAs) are small non-coding RNAs that posttranscriptionally regulate the expression of protein-coding genes. The discovery of miRNAs has resulted in a paradigm shift in our knowledge about gene control and therapeutic intervention. Through their binding to their target genes, these “master regulators” induce subtle alterations in gene expression that can culminate in major phenotypic changes. This is based on the notion that miRNAs are pleiotropic, referring to the fact that miRNAs can bind to and affect multiple targets. Although the expression of an individual miRNA target may only change marginally, the combined effect of suppressing several targets at the same time results in a phenotypic transformation. This is most clearly illustrated in the context of cancer where miRNA dysregulation contributes to many types of cancer. In some instances the combination of multiple subtle changes causes the tumor cells to become addicted to a single miRNA. MiR-21 and miR-155 are two oncogenic miRNAs (oncomiRs) that have shown this type of addictive pattern in vivo. Similarly loss of key tumor suppressive miRNAs, through epigenetic silencing, genomic loss, and reduced upstream signaling and processing, has been correlated with disease state. Based on this knowledge we have embarked on three very distinct yet equally important areas of miRNA therapeutic biology. Project 1: Using tumor suppressive miRNAs as cancer therapeutics and sensitizers Project 2: Restoring let-7 processing through small molecule discovery Project 3: Identification of miRNAs that potentiate KRAS- and/or p53- driven lung tumorigenesis Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Kazemian | Majid | Biomolecular Structure and Biophysics, Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Majid KazemianCurrent Research InterestsWe are interested in studying underlying mechanism of autoimmune diseases such as rheumatoid arthritis, type I diabetes and asthma and infections including hepatitis and Epstein-Barr viruses leading to cancer. Our lab integrates computational and high throughput experimental approaches (e.g. RNA-seq, ChIP-seq, ATAC-seq, Hi-C) in to identify novel pathways, new host-pathogen interactions and regulatory noncoding RNAs (e.g. enhancer RNAs and circular RNAs) that could trigger disease. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactKazemian@purdue.edu
 |
| Kenttamaa | Hilkka | Chemical Biology |
Hilkka KenttamaaProfessor—Analytical Chemistry and Organic Chemistry Current Research InterestsWe are interested in various aspects of organic and bioorganic mass spectrometry. The focus areas include fundamental understanding of gas-phase ion chemistry and structural characterization of molecules. Mass spectrometry provides a powerful tool for the analysis of organic and biomolecules. Chemical ionization methods are developed for (1) distinction of isomeric compounds, (2) structural characterization of complex molecules, such as drug metabolites and peptides, and (3) examination of the composition of complex mixtures, especially degraded biomass intended for conversion to biofuels. Laser-based methods are developed for evaporation of intact neutral biopolymers into mass spectrometers. Mass spectrometry allows the direct examination of organic and biological reaction intermediates that are difficult to study by other experimental approaches. The information obtained on the molecules' intrinsic properties provides the basis for understanding of their behavior in solution. This work includes the design of gas-phase synthesis strategies, kinetic and thermochemical studies, and molecular orbital calculations on aromatic radicals and biradicals, nucleotide radical cations and peptide radical cations. The knowledge obtained in these studies aids the understanding of DNA and protein degradation by antitumor drugs and UV irradiation. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthilkka@purdue.edu  |
| Kerstein | Patrick | Integrative Neuroscience |
Patrick KersteinAssistant Professor Current Research InterestsOur research is focused on identifying the genetic mechanisms that control neuronal cell fate and connectivity in the developing visual system in health and disease. To identify these genes, we use mouse genetics for cell-type specific gene knockouts and single cell labeling for cell fate mapping and morphological analysis. Using these techniques, we are interested in how the developing retina generates the correct neuronal cell types and synaptic connections. We are also interested identifying the environmental factors, such as Pb toxicity, that lead to visual disorders, such as retinal degeneration and optic nerve atrophy. The goal is to identify and develop novel therapeutic targets to prevent and repair visual diseases.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactpkerstei@purdue.edu  |
| Kessler | Sharon | Plant Biology |
Sharon KesslerAssociate Professor of Botany and Plant Pathology Current Research InterestsThe Kessler Lab studies the molecular mechanisms that control pollination. Our main focus is cell-cell communication between the male and female tissues, specifically how do the female cells known as the synergids “talk” to the pollen and tell it to burst and release the sperm cells so that double fertilization can occur to produce viable seeds. This signaling pathway shares interesting parallels with plant-pathogen interactions indicating common mechanisms for perceiving both beneficial and harmful invaders. Our lab focuses on a plant-specific family of 7 transmembrane proteins called MLOs which play a role in various signaling processes ranging from pollen tube reception to pathogen infection. Our goal is to understand how receptor-kinase triggered changes in cell polarity and MLO protein accumulation patterns lead to successful reproduction and development. We use genetics, molecular biology, cell biology, and live imaging in the model plant Arabidopsis thaliana to study these important signaling molecules.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkessles@purdue.edu  |
| Kihara | Daisuke | Biomolecular Structure and Biophysics, Computational and Systems Biology |
Daisuke KiharaProfessor, Department of Biological Sciences and Department of Computer Science Current Research InterestsProtein Bioinformatics. Our lab develops and applies computational tools for building protein 3D structure models, protein complexes, structure modeling for cryo-electron microscope density data, drug screening, and protein function prediction. We use various machine learning and computational biophysics approaches in the tools. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdkihara@purdue.edu  |
| Kimbrough | Adam | Computational and Systems Biology, Integrative Neuroscience |
Adam KimbroughAssistant Professor Current Research InterestsOur laboratory is currently interested in understanding how the brain is changed after substance use or neurodegenerative diseases. We are interested in how addiction forms, and alters the brain, leading to motivation for excessive drug intake. Current projects include: 1) determining critical brain regions involved in alcohol drinking, 2) assessing how oxycodone use alters brain structure and function, 3) modeling polysubstance use of alcohol and oxycodone, and 4) determining the potential for alcohol use to increase the risk of neurodegenerative diseases such as Alzheimer's disease.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkimbroua@purdue.edu  |
| Kim | Taeyoon | Biomolecular Structure and Biophysics, Biotechnology |
Taeyoon KimAssistant Professor/Weldon School of Biomedical Engineering Current Research InterestsThe previous focus of my work has been on the development and use of computer simulations to address fundamental questions about the mechanics and dynamics of passive actin cytoskeleton. I developed a state-of-the-art agent-based model for actin cytoskeleton that computes macroscopic dynamics from realistic nano-scale representations of cytoskeletal constituents. Using the model, I have shown how the mechanical and dynamic properties of the cytoskeletal components at a microscopic scale determine the rheological behaviors of the actin cytoskeleton at a macroscopic scale. These studies have revealed key mechanistic features of actin cytoskeleton that current experimental approaches have been unable to illuminate. My lab has recently focused on elucidating mechanisms of actomyosin contractility using the computational models. We incorporated molecular motors in the previous model with reflection of realistic mechanochemistry of myosin II motors. We have shown how forces are generated in disorganized actomyosin structures such as cortex-like networks and bundles and how actomyosin contractility helps cells sense the rigidity of surrounding environments. Research areas in which my lab is interested for a longer term are various biological processes at cellular and tissue scales. Currently, we are developing models for cell migration, mechanotransduction, and early-stage vasculogenesis. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Kinzer- Ursem | Tamara | Computational and Systems Biology, Integrative Neuroscience |
Tamara Kinzer- UrsemAssistant Professor of Biomedical Engineering Current Research InterestsOur research interests lie at the intersection of engineering, systems biology and biochemistry. We implement a combined theoretical-experimental approach to quantitatively study the dynamic regulation of protein networks that drive cellular behaviors. Our work can be characterized as tools development for studying protein function and signaling dynamics. We use protein engineering techniques to developing new methods to study protein signaling in high throughput formats. These in vitro techniques are complemented by development of in silico mathematical representations of protein signaling that allows us to understand protein function in the context of larger signaling networks and to probe network function in the context of overall cellular behaviors. These techniques are being applied to study calcium-dependent signaling in excitatory cells and the dynamic regulation of protein phosphorylation via kinase and phosphatase proteins. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Kinzig | Kimberly | Integrative Neuroscience |
Kimberly KinzigAssociate Professor of Psychological Sciences Current Research InterestsResearch in my laboratory is focused on neural and endocrine factors involved in the control of food intake and regulation of energy balance. We are currently investigating the role of dietary macronutrients in energy balance and also how chronic caloric restriction influences regulatory responses to food intake. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactkkinzig@purdue.edu  |
| Kirchmaier | Ann | Cancer Biology, Microbiology, Immunology and Infectious Diseases |
Ann KirchmaierAssociate Professor of Biochemistry Current Research InterestsThe research in my lab focuses on understanding how cells regulate chromatin and epigenetic processes. Our research examines the role of metabolism, the cell cycle and DNA replication in assembly or maintenance of chromatin structures and the effects of these structures on DNA replication and DNA repair. We are interested in how the cell restricts heterochromatin to specific genomic loci, why heterochromatin formation is regulated by the cell cycle, how transcription of genes is prevented in silenced regions, and whether epigenetic processes are influenced by or influence events including DNA damage and the initiation of DNA replication. We are intrigued by how epigenetic states are maintained throughout the cell cycle and are duplicated and inherited each time the chromosome itself is replicated and the cell divides. We investigate how environmental factors perturb epigenetic processes and can contribute to inappropriate gene expression, developmental defects, tumorogenesis and other catastrophic disorders. Our laboratory combines genetic, molecular biology, biochemical, and quantitative microscopy-based approaches with mammalian cell culture and yeast genetics to understand the impact of genetic and external factors on chromatin modifications and epigenetic gene regulation. Silencing in Saccharomyces cerevisiae: We use silent chromatin in S. cerevisiae as a model for understanding how epigenetically regulated chromatin structures are established, maintained and inherited. Silenced chromatin in budding yeast, is akin to heterochromatin in organisms such as maize, flies, and mammals. S. cerevisiae uses epigenetically inherited chromosomal structures to regulate a variety of cellular activities including controlling cell-type specific gene expression, modulating ribosomal RNA levels, and preserving telomere structure and stability. Silenced regions in S. cerevisiae that are regulated, in part, by the Silent Information Regulator, or Sir, proteins include the silent mating-type loci, HML and HMR, the rDNA locus and the telomeres. To mediate silencing at a given site on a chromosome, an organism must first have a way to recruit the proteins that compose silenced chromatin to that locus. In yeast, regulatory sites known as silencers flank the silent mating-type loci. Silencers contain binding sites for the Origin Recognition Complex (ORC), and the transcriptional regulators Rap1p and Abf1p. In addition, the Sir proteins, Sir1p, Sir2p, Sir3p and Sir4p, are structural components of silenced chromatin in yeast. Unlike ORC, Rap1p and Abf1p, however, the Sir proteins do not bind to DNA site-specifically. Instead, Sir proteins associate with silencers through protein-protein interactions between each other, proteins bound at silencers, and histones H3 and H4. Once initiated, silencing spreads along the chromosome over several kilobases of DNA and inactivates gene expression. Once established, the transcriptionally inactive state of this region of the chromosome is maintained throughout the cell cycle, is copied faithfully during DNA replication and can be stably inherited in subsequent cell generations. Many proteins involved in silencing in yeast have homologs in a wide variety of organisms, including humans where several are involved in development and differentiation or have been implicated in cancer. Epigenetic Processes and Replication-Coupled Chromatin Assembly: We are investigating how a network of chromatin assembly factors, histone-modifying enzymes and replication factors interact to assemble appropriately modified nucleosomes during DNA replication and thereby promote epigenetic processes and genome integrity. We have been characterizing how silencing is affected by the DNA polymerase processivity factor PCNA through pathways involving several factors including the chromatin assembly factors, PCNA loading complex RF-C and histone acetyltransferases. As nucleosomal DNA serves as the foundation upon which silent chromatin is built, perturbations in replication-coupled chromatin assembly can alter the efficiency and location of silent chromatin formation as well as lead to defects in maintaining and inheriting epigenetic states. We are also exploring how repair of DNA damage can be compromised in mutants with defects linked to histone modifications and replication-coupled chromatin assembly pathways. Influences of Metabolism on Chromatin: Genome integrity is fundamental to the health of an organism, and, when disrupted, can lead to cancer. We are uncovering how metabolite availability influences chromatin-regulated processes, including cell survival during DNA replication stress. We have discovered that accumulation of the metabolite fumarate, or loss of TCA cycle enzyme fumarase (ortholog of the tumor suppressor FH), promote resistance to replication stress, in part, by inhibiting histone demethylation, and bypassing requirements for proteins involved in processing or restarting stalled replication forks. Defining the Functional Composition of Chromatin: The simplest structural unit of chromatin, the nucleosome, can exist in a variety of configurations depending on the histone variants and post-translational modifications present. The composition of individual nucleosomes influences chromatin structure and function and provides signals to the cellular machinery to promote gene activation or repression. These signals tend to be dynamic and can vary as a function of the cell cycle or growth conditions. Yet, our understanding of the patterns found within individual nucleosomes and presented to the cellular machinery is limited. Population-based approaches widely used to characterize chromatin composition have been valuable in identifying and mapping individual modifications within histones, but have been limited in describing which modifications are combined within the same nucleosomes. As a powerful complement to standard approaches, we are utilizing single molecule strategies in vitro and in single mammalian and yeast cells to probe epigenetic processes that regulate transcriptional states, and responses to DNA damage in several collaborative projects. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkirchmaier@purdue.edua
 |
| Knipp | Gregory | Chemical Biology, Integrative Neuroscience, Microbiology, Immunology and Infectious Diseases |
Gregory KnippAssociate Professor Current Research InterestsMy laboratory is dedicated to the utilization of new and improved models for assessing pharmaceutical performance. The laboratory's research interests include preclinical in vitro and in vivo ADMET screening, physicochemical characterization, early formulation development, and performing pharmacokinetic studies of new candidates and traditional chemical entities. Recently we have focused on the development of a novel, direct contact human blood brain barrier triculture model for screening permeability-linked neuronal response. We have also been collaborating on projects focused on the development of compounds that mitigate viral infections. In addition, I currently serve as the faculty directors of the Purdue Translational Pharmacology (PTP) CTSI Core Facility, where we conduct preclinical pharmacokinetic testing in rodent and the porcine animal models under stress free conditions. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactgknipp@purdue.edu  |
| Konradt | Christoph | Microbiology, Immunology and Infectious Diseases |
Christoph KonradtAssistant Professor Current Research InterestsResearch Focus 1: The role of EC in the immune response There are more than 10 trillion EC in the human body, that cover 4000 m2, and these are an important replicative niche for a subset of micro-organisms such as Dengue virus, West Nile Virus, CMV, S. aureus and T. gondii. For many micro-organisms the ability to infect EC is a key part of their pathogenesis. Indeed, EC express TLR and influence coagulation and neutrophil recruitment and their activation can lead to vascular damage, while expression of adhesion molecules promotes extravasation of inflammatory cells. Numerous in vitro studies have demonstrated that EC can be activated by cytokines to limit viral, bacterial and parasite replication. Furthermore, evidence that EC interact with the adaptive response is implicit in the ability of EC to present MHC class I & II restricted antigens. However, whether EC antigen presentation via MHC-I or MHC-II has a tolerogenic or inflammatory role during infection remains unclear. Therefore, there is a major knowledge gap in our understanding in the involvement of EC in orchestrating a T cell response or how the immune system reacts to infected EC in vivo. Research Focus 2: Immune responses at the Maternal-Fetal Interface The maternal-fetal interface is the interface between the uterine mucosa and the extraembryonic tissues of the developing conceptus. The placenta functions as the primary nutrient and gas exchange organ of the fetus by diverting maternal blood flow from the uterus. Once inside the placenta, maternal blood exchanges nutrients, gases, and metabolic waste products with fetal blood coursing through a physically separate vasculature that connects to the fetus via the umbilical cord. A successful pregnancy involves complex interactions between fetal trophoblasts, endothelial cells and maternal decidual immune cells, creating an immunologically unique site that allows the tolerance to the allogenic fetus but still maintains host defense against possible pathogens. Infections are a well-described cause for fetal losses and stillbirths, as well as for perinatal morbidity. Indeed, infections are accounted for up to 15% of early miscarriages and up to 66% of late miscarriages. Moreover, infections can further lead to preterm birth or infants with birth defects leading to lifelong disabilities. This is accompanied with significant health care costs and a burden for the mothers mental health and psychological wellbeing. Immune responses at the maternal-fetal interface during infection are poorly understood and there is a real need for a better understanding of the immunological mechanisms at this site. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactckonradt@purdue.edu  |
| Krishna | Jayant | Biomolecular Structure and Biophysics, Integrative Neuroscience, Membrane Biology |
Jayant KrishnaAssistant Professor Current Research InterestsWe are interested in elucidating the fundamental biophysical mechanisms by which single neurons process and integrate information, and how these features influence and decide population coding, plasticity, and sensory input processing in the brain. To this end, we focus on the computational role that synapses and dendrites play in deciding and influencing the overall input-output transfer function of a neuron. There is growing evidence to suggest that the location and interplay between synapses, local dendritic electrical features, spatial arrangement of specific membrane channels, and local biophysical properties strongly influence local and global computations (Ex: dendritic spiking, co-incidence detection, electrical compartmentalization, biochemical compartmentalization, and simple logic functions). Yet, exactly how these multiple integrative effects influence overall circuit output and create a stable behavioral response is still unknown. We seek to develop new tools and techniques, and in conjunction with cutting-edge two-photon imaging and nanoscale electrophysiology tease apart these fundamental biophysical mechanisms through which single neurons and small populations of neurons process and shape information
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkjayant@purdue.edu
 |
| Krishnan | Ananthanarayan | Integrative Neuroscience |
Ananthanarayan KrishnanProfessor Current Research InterestsProfessor Krishnan is an auditory electrophysiologist Audiologist who joined the Purdue faculty in the fall of 1998. His research and teaching interests are concentrated in the area of auditory physiology. Specifically, Dr. Krishnan is interested in investigating electrophysiological correlates of various psychoacoustic phenomena in both normal and hearing-impaired individuals. His current research involves the evaluation of neural encoding of speech-like sounds in normal and hearing impaired individuals. Professor Krishnan holds the Certificate of Clinical Competence in Audiology and is a member of the Association for Research in Otolaryngology. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Krusemark | Casey | Biotechnology, Cancer Biology, Chemical Biology |
Casey KrusemarkAssistant Professor of Medicinal Chemistry and Molecular Pharmacology Current Research InterestsResearch in the Krusemark laboratory is focused on the discovery of functional small molecules using the power of in vitro evolution. DNA-programmed combinatorial chemistry (DPCC) enables the "translation" of DNA constructs into DNA conjugates with wholly synthetic small molecules, thus enabling Darwinian-like selections. DPCC allows the synthesis and functional interrogation of small molecule libraries of unprecedented size and complexity (presently up to 1010 members). The Krusemark lab works on the further development of the DPCC technology, towards the goal of a streamlined system for generating drug leads and biological tool compounds. We use a combination of chemical, engineering, and molecular biology methods. We are working to expand the synthetic toolbox available for building molecules on unprotected DNA towards drug-like small molecules. Our lab will develop tools to study the protein kinase C (PKC) family of kinases, which are involved in the signal transduction pathways of numerous and varied cellular processes. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Kuang | Shihuan | Cancer Biology, Chromatin and Regulation of Gene Expression, Microbiology, Immunology and Infectious Diseases |
Shihuan KuangProfessor Current Research InterestsSkeletal muscle and adipose tissue stem cells, regeneration, muscular dystrophy, obesity, type 2 diabetes, aging. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactskuang@purdue.edu  |
| Kuhn | Richard | Biomolecular Structure and Biophysics, Microbiology, Immunology and Infectious Diseases |
Richard KuhnTrent and Judith Anderson Distinguished Professor of Science, Department of Biological Sciences, Kreniki Family Director, Purdue Institute of Inflammation, Immunology and Infectious Disease Current Research InterestsOur research focuses on understanding the biology of viruses that infect humans. Specifically, my lab is interested in viruses that contain a lipid bilayer membrane and contain plus strand RNA as their genetic material. These viruses are grouped in the Togaviridae and Flaviviridae families; some representative members include Sindbis, Ross River and Venezuelan equine encephalitis virus for Togaviruses, and yellow fever, dengue, West Nile and hepatitis C virus for the Flaviviruses. Viruses within these two groups pose significant risks to large segments of the population, and methods for controlling infection and disease are few. Our goal is to understand all aspects of their replication cycle at the molecular level by integrating techniques from molecular genetics, biochemistry and structural biology. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactkuhnr@purdue.edu  |
| LaCount | Doug | Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Doug LaCountAssociate Professor Current Research InterestsThe central theme of my research program is the discovery and characterization of protein interaction networks between intracellular pathogens and their host cells. We seek to understand how intracellular pathogens manipulate host cells, how host cells respond to intracellular pathogens, and how this information can be exploited to develop new antimicrobial or antiviral drugs. We are interested in developing new host-pathogen protein interaction networks, comparing host-pathogen interaction networks from different organisms, and performing in-depth analyses of the roles of host proteins in virus and parasite lifecycles. Our research primarily focuses on two mosquito-transmitted pathogens that are major global health problems: the malaria parasite Plasmodium falciparum and dengue virus. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdlacount@purdue.edu  |
| Laskin | Julia | Biomolecular Structure and Biophysics, Biotechnology, Cancer Biology, Integrative Neuroscience, Membrane Biology |
Julia LaskinProfessor Current Research InterestsMy group is developing state-of-the-art instrumentation for molecular imaging of lipids, metabolites, and proteins in biological samples using mass spectrometry. In particular, we have developed a novel ionization method (nano-DESI), which enables sensitive and quantitative imaging of hundreds of biomolecules in tissue sections with high spatial resolution. We also develop tools for imaging isomeric lipids and metabolites, which cannot be distinguished based on their mass alone. The experimental techniques are combined with deep learning and dimensionality reduction tools to obtain a concise representation of the vast experimental data. We apply these tools to both healthy and diseased mammalian tissues to explore molecular signatures of aging, neurodegenerative diseases, embryo implantation in healthy and aberrant pregnancy, and other processes. We are involved in many productive collaborations with biologists who provide us with relevant samples.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjlaskin@purdue.edu  |
| Leung | Yuk Fai | Computational and Systems Biology, Integrative Neuroscience |
Yuk Fai LeungAssociate Professor in Biological Sciences Current Research InterestsDrug discovery for retinal degeneration Retinal degeneration is a group of inherited eye diseases including retinitis pigmentosa and age-related macular degeneration that impair our vision. Although much has been learned about the molecular basis of these diseases, they are still incurable. To expedite discovery of new drugs for these diseases, our group at Purdue University studies zebrafish retinal-degeneration models. We screen drug libraries on these models using high-throughput visual-behaviour assay. We develop novel statistical and machine-learning tools to analyze the large-scale behavioural data, and to to determine which drugs helped the retinal-degeneration models. We then study how the positive drugs work at molecular, genomics and cell-biology levels. Please visit our lab website for the most updated information.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyleung@purdue.edu  |
| Lill | Markus | Chemical Biology, Computational and Systems Biology |
Markus LillAssistant Professor Current Research InterestsDevelopment of Novel Concepts for Computer-Aided Drug Discovery In the last few decades, computer-aided drug discovery (CADD) concepts have matured into powerful tools for identifying and optimizing lead structures. Based on the three-dimensional structure of target proteins, structure-based design has become a widespread approach to identify potential drug candidates in silico. While CADD techniques have been widely used to attain a qualitative understanding of ligand binding to proteins, a current challenge is to quantify their interaction. Incorporation of Protein Flexibility and Quantification of Binding Affinities To compute the binding affinity of a given compound, an accurate prediction of relative free energies of binding, e.g. by using free energy perturbation calculations, would seem to be the method of choice. Unfortunately, the associated procedures are computationally demanding and limited to the comparison of affinities of structurally rather similar compounds. On the other hand, automated docking, as used for virtual screening, is up to now limited in its potential to reliably predict binding affinities. One major drawback is the underlying rigid-receptor assumption of most of these methods: the protein conformation remains unaltered during the docking process and can not adapt its shape and properties to the ligand binding to it. X-ray crystallography, however, has clearly demonstrated the importance of induced fit upon ligand binding. To face this problem, we recently contributed to the development of a novel concept combining automated docking and multidimensional QSAR to identify feasible binding modes of protein-bound molecules and to compute their binding affinity. During the docking process protein flexibility is accounted for by allowing the side chains of the amino acids to adapt to the compound binding to it. Multidimensional QSAR (e.g. software Quasar and Raptor) allows to represent each ligand molecule as an ensemble of conformations, orientations, stereoisomers and protonation states (4D-QSAR), thereby reducing the bias in identifying the bioactive conformer. In addition, it explicitly simulates induced protein fit. This concept was successfully applied in lead optimization projects as well as for the estimation of the toxic potential of drugs and chemicals. In current research, we focus on the aspects of solvation, entropy, and especially protein flexibility, which are only partially included in our current technologies. Our research philosophy is to use MD simulations aiming to obtain detailed data about these aspects of ligand-protein interaction, and to translate this information into empirical models. The latter are implemented in novel computational concepts for efficient use in CADD. These new methods as well as the already developed tools are utilized to the following areas of application. Adverse Reactions of Drugs and Chemicals An analysis of the main reasons for attrition in drug development showed that more failures are attributed to poor pharmacokinetics, animal toxicity and adverse effects in man than due to lack in efficacy. Thus, a fast and efficient prediction of adverse reactions of drugs as early as in the design phase of synthesis of compounds by computational means becomes desirable. One important molecular mechanism triggering adverse reactions is due to the binding of small molecules to specific receptors or enzymes. Molecules may share pharmacophoric properties similar with the endogenous compound, thus, simulating the biochemical effect activated by the very receptor. Molecules can also trigger the inverse physiological result, agonism or antagonism. For example, drugs and chemicals in our environment can bind to nuclear receptors and induce or inhibit the transcription processes, resulting in endocrine disrupting effects (e.g. development and reproduction toxicity; cancer). Various simulation studies on estrogen, androgen and thyroid receptor have shown that our concept is able to predict binding affinities of structurally diverse sets of compounds, and thus to estimate receptor-mediated toxicity. Inhibition of cytochrome P450 (CYP) enzymes by small molecules represents a major mechanism associated with undesired drug-drug interactions responsible for a substantial number of late-stage failures in the pharmaceutical drug development process. For a quantitative prediction of associated pharmacokinetic parameters, we developed a computational model for CYP3A4, allowing us to predict the inhibitory potential of structurally diverse molecules. This specific enzyme is responsible for metabolizing more than 50% of the marketed drugs. Lead Optimization for GPCRs We are further interested in optimizing new lead candidates for GPCRs. As no experimental data on the 3D structure of the protein is available, we identify the conformation of the ligands and their relative orientation by maximizing the similarity of the physico-chemical properties of the compounds in 3D space using the Symposar concept. Symposar aligns ligands utilizing fuzzy-like 2D-subfeature mapping and, subsequently, a Monte-Carlo search on a 3D similarity grid. The resulting ensemble of binding conformations has been used as input for the multidimensional QSAR technologies, to quantify the binding energy. Applying this concept to predict the binding affinity of 186 molecules for the Bradykinin B2 receptor by means of the Quasar and Raptor technologies allowed for consensus scoring and generated topologically and quantitatively consistent receptor models. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmlill@purdue.edu  |
| Lim | Seong-Oe | Cancer Biology, Microbiology, Immunology and Infectious Diseases |
Seong-Oe LimAssistant Professor
Current Research InterestsResearch in the Lim lab focuses on identification of regulatory mechanism of anti-tumor immunity in several types of cancers in particular breast cancer. Based on the mechanism, we focus on developing strategies that enhance therapeutic efficacy of immunotherapy and identification of new druggable target to overcome immune checkpoint blockade resistance. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactlimsoe@purdue.edu  |
| Long Lin | Tsang | Microbiology, Immunology and Infectious Diseases |
Tsang Long LinProfessor of Veterinary Pathology Current Research InterestsPathology, pathogenesis, immunology/immunopathology, and vaccinology of infectious, toxic, and neoplastic diseases PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contacttllin@purdue.edu  |
| Lindemann | Stephen | Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Stephen LindemannAssistant Professor of Food Science and Nutrition Science Current Research InterestsBroadly, Prof. Lindemann’s interests lie in identifying the principles that govern structure-function relationships in microbial communities and how host-microbe and microbe-microbe interactions govern their dynamics and emergent properties. Specifically, he is focused on using molecular microbial ecology techniques to understand how microbes compete for - and cooperate in - degradation of complex substrates and how this division of metabolic labor influences gut microbiome fermentation of dietary fiber polysaccharides. We are interested in how differences in physical and chemical structures influence gut microbiome structure and function, and how structural differences influence health outcomes. Finally, we are interested in how interactions between beneficial microbes exclude pathogenic organisms and modulate inflammation in the colon. Our research employs genome-enabled systems biology (“-omics”) approaches coupled with genetic techniques, computational biology, and modeling. We envision a world in which dietary solutions can be reliably employed to improve nutrition and reduce acute and chronic gut illness.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactlindems@purdue.edu  |
| Lipton | Mark | Chemical Biology |
Mark LiptonAssociate Professor — Organic Chemistry Current Research InterestsOur research effort combines the disciplines of organic synthesis, bioorganic chemistry and molecular modeling. Current projects in the Lipton group fall into two areas: Development of novel synthetic methodology.We have recently developed two new reagents for the guanylation of amines, an important and underdeveloped reaction. We also have developed a novel, cyclic dipeptide catalyst (1) for an asymmetric variant of the Strecker amino acid synthesis. This has led to a broad effort directed toward the use of cyclic dipeptide catalysts (e.g., 2) in asymmetric carbon-carbon bond forming reactions. We have initiated another broad effort in the area of reactions on solid supports. We began with the synthesis of cyclic dipeptides 1 and 2 for our catalysis studies. Recent projects involve the synthesis of peptidomimetics (e.g., 3) and macrocyclic lactams on solid supports. Design and synthesis of biologically active molecules.Ongoing projects include the synthesis of inhibitors of the enzymes cyclophilin A and HIV-1 protease (both essential for the replication of the HIV-1 virus and the pathogenesis of AIDS), and novel DNA-cleaving agents for the treatment of cancer. Projects of this type usually are designed using molecular modeling and tested "in house" after synthesis
PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactlipton@purdue.edu  |
| Lisch | Damon | Chemical Biology. Plant Biology |
Damon LischAssociate Professor Current Research InterestsDr. Lisch is interested in the regulation and evolution of plant transposable elements and the role that transposable elements have played in plant evolution. Transposable elements, or transposons, are, by far, the most dynamic part of the eukaryotic genome, and the majority, often the vast majority, of plant genomes are composed of these genomic parasites. Although they are an important source of genetic novelty, transposons can also be a significant source of detrimental mutations. Because of this, plants (and indeed all eukaryotes) have evolved a sophisticated “immune system” whose function is to detect and epigenetically silence them. Dr. Lisch’s research centers on determining the means by which transposons are detected and then maintained in a silenced state and the effect that this process has had on the trajectory of plant evolution. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdlisch@purdue.edu  |
| Li | Tonglei | Biomolecular Structure and Biophysics, Biotechnology, Computational and Systems Biology |
Tonglei LiProfessor Current Research InterestsMultiscale Modeling and Simulation for Drug Development: Over the last few years, we have integrated molecular modeling and simulation with continuum dynamics and pharmacokinetics modeling. For example, one area is to simulate local drug transport and absorption with fluid dynamics and predict system exposure of drug molecules. Another study is to investigate protein stability under hydrodynamic stress. Our research interests lie in integration of macro-, meso-, and microscale modeling and simulation methods for understanding the in vivo fate of drug products. Deep Learning for Drug Discovery and Development: We have exploited the hard and soft acids and bases principle (HSAB) within the conceptual density functional theory (CDFT) framework to characterize the locality and strength of intermolecular interactions. The efforts have led to our recent development of a novel concept to depict molecules in the context of molecular interaction. It allows us to utilize deep learning and develop predictive models of molecular properties and functionalities. The concept also permits de novo design of molecules. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacttonglei@purdue.edu  |
| Liu | Julie | Cancer Biology |
Julie LiuAssociate Professor Current Research InterestsProf. Liu's research focuses on developing protein-based biomaterials for application in tissue engineering, regenerative medicine, and surgical glues. One area of investigation is in designing biomaterials from recombinant proteins. Recombinant proteins have molecular-level control over amino acid sequence, a property that dictates the subsequent structure and function. To fully utilize the potential of these biomaterials, her work focuses on establishing key sequence-structure-function relationships between protein design and the resulting material properties. Another area of investigation is the use of natural proteins, such as collagen, and other important matrix molecules, such as hyaluronic acid and chondroitin sulfate. In addition to being biomimetic, these materials contain important biological cues that govern cell response. Overall, the Liu group utilizes both recombinant proteins and natural matrix molecules to design materials with specific performance capabilities for cartilage engineering, in vitro tissue models for drug testing, and biomedical adhesives. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjulieliu@purdue.edu  |
| Liu | Biomolecular Structure and Biophysics, Chemical Biology, Plant Biology |
Xing LiuAssistant Professor
Current Research InterestsWe are interested in understanding the mechanism of protein ubiquitination and how this post-translational modification determines proper cellular and organismal functions. To achieve this goal, we use human cells and plants as our experimental systems. Our current research focuses on the dynamics of Skp1•Cul1•F-box protein (SCF) ubiquitin E3 ligases, a group of enzymes that play critical roles in eukaryotic biology, ranging from cell division to organ formation. Each SCF has a core Cul1 protein that assembles with a family of F-box proteins to form different complexes with distinct substrate specificities. The genome of human and Arabidopsis thaliana encodes 69 and ~700 F-box proteins, respectively, each of which needs to assemble with Cul1 for the degradation of its substrates. Therefore, recruiting the right type of F-box protein at the right time in the right location is essential for proper SCF function. We aim to understand how the cellular repertoire of the SCF is dynamically controlled to meet the changing needs of substrates, applying multidisciplinary approaches such as biophysics, chemical genetics, genome editing, quantitative mass spectrometry, and computational modeling. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactxingliu@purdue.edu
 |
|
| Liu | Zhongming | Biomolecular Structure and Biophysics, Biotechnology, Integrative Neuroscience |
Zhongming LiuCurrent Research InterestsMagnetic Resonance Imaging Neural Engineering Neuroscience Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactzmliu@purdue.edu  |
| Li | Ying | Computational and Systems Biology, Plant Biology |
Ying LiAssistant Professor of Horticulture Current Research InterestsMy lab is interested in studying how crop metabolic networks respond to environmental and developmental stimuli at transcriptional and chromatin levels. Understanding these regulatory systems is vital to agriculture productivity and environmental sustainability. We apply cutting-edge molecular tools and computational approaches to probe gene regulatory networks underlying environmental and developmental responses. We harness the power of omics to develop a comprehensive understanding of plant responses at both transcriptional and epigenetic levels. Our ultimate goal is to identify key molecular players including epigenetic regulators to engineer better crops to promote agricultural sustainability and productivity in a changing environment. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactli2627@purdue.edu  |
| Lohman | Jeremy | Biomolecular Structure and Biophysics, Chemical Biology |
Jeremy LohmanAssistant Professor of Biochemistry Current Research InterestsNatural products are one of our best sources of drugs and drug leads. Natural products from bacteria and fungi have the benefit of being supplied through fermentation, in contrast to sources such as plants and sponges that suffer from slow-growth and ecological concerns such as overharvesting. Natural products exist to benefit their hosts, as such some natural product drugs are not ideal for human use. Synthetic derivatives of these natural products often have superior activity, but cost significantly more to produce. Therefore finding methods to generate natural product derivatives through fermentation is an important goal. Combinatorial biosynthesis is the process of creating natural products through the genetic engineering of producing organisms to generate natural product derivatives. Currently, the easiest method for producing natural product derivatives is through deletion of genes encoding enzymes that add functionality to a natural product. However, many enzymes are essential to building the core of a natural product, limiting the number of derivatives created through gene deletion. Another approach is to add enzymes to the pathway or to alter the substrate specificity of enzymes within the biosynthetic pathway. The major hurdle to the second approach is a lack in our current understanding of the structure-function relationships of these enzymes. Our lab is seeking to understand the sequence-structure-function relationships in families of biosynthetic enzymes, so that our knowledge will be of use in engineering multiple biosynthetic pathways. Through reverse engineering the sequence-structure-function relationships of biosynthetic enzyme families we will engineer new substrate specificity into enzymes within pathways, and thus enable true combinatorial biosynthesis. Using bioinformatics, x-ray crystallography and enzymology together, we will discover how sequence-structure-function is related within families of biosynthetic enzymes. We will have genes synthesized that encode proteins with engineered substrate specificity and probe their activities in vitro. Finally using genetics we will introduce the engineered synthetic genes into natural product producers to isolate natural product derivatives. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjlohman@purdue.edu  |
| Low | Philip | Cancer Biology, Chemical Biology, Microbiology, Immunology and Infectious Diseases |
Philip LowRalph C. Corley Distinguished Professor Current Research InterestsWe have developed methods to target drugs specifically to diseased cells, thereby avoiding collateral toxicity to healthy cells. To achieve this specificity, we have designed low molecular weight targeting ligands that bind selectively to pathologic cells and have linked them to the desired therapeutic or imaging agents. In the case of cancer, we have exploited the up-regulation of folate receptors on ~40% of human cancers to target the following pharmaceuticals to tumor masses: i) cytotoxic drugs, ii) protein toxins, iii) miRNA and siRNA constructs, iv) fluorescent dyes for fluorescence-guided surgery, v) radioimaging agents, vi) CAR T cells, vii) liposomes with entrapped drugs, viii) radiotherapeutic agents, ix) immunotherapeutic agents, and x) enzyme constructs for prodrug therapy. More recently, we have developed novel targeting ligands for most other cancers and tumor stromal cells, including regulatory T cells, MDSCs, tumor associated macrophages, and cancer associated fibroblasts (CAFs). In the course of these latter studies we have found that reprogramming of tumor stromal cells can have a profound impact on tumor growth and survival. Finally, we have also developed targeted therapies for many other diseases, including inflammatory diseases (rheumatoid arthritis, psoriasis, multiple sclerosis, ulcerative colitis, atherosclerosis, Crohn’s disease, sarcoidosis, systemic lupus erythematosus, etc.), viral infections (HIV, hepatitis B, influenza, etc.), fibrotic diseases (cirrhosis of the liver, idiopathic pulmonary fibrosis, scleroderma, etc.), inherited diseases (sickle cell disease, etc.), CNS diseases (Parkinson’s disease, Alzheimer’s disease, autism, etc.), and traumatized tissues. By concentrating the healing power solely in the cells that need it, we reduce/eliminate off-target toxicity while improving on-target efficacy. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactplow@purdue.edu  |
| Low-Nam | Shalini | Biomolecular Structure and Biophysics, Chemical Biology |
Shalini Low-NamAssistant Professor Current Research InterestsOur group is focused on discovering molecular mechanisms of cellular decision-making. We have introduced a molecular impulse-response function to map the complete history of input accumulation (with single molecule precision), through signal integration, and up to the outcome, in the form of a cellular behavioral response. We focus on understanding how single cells satisfy activation thresholds, in order to develop strategies to modulate these responses. We work on both tumor (breast and pancreatic) and immune cells (T cells and macrophages) in the complex cancer microenvironment. Many key impulses are detected at interfaces between these cells; thus, we make quantitative measurements of the dynamic landscape (biophysical features) of intercellular spaces that modulate signal propagation. We use high resolution microscopy to directly observe the impulse-response function and combine in vitro reconstitution and live cell approaches to understand key activation mechanisms (growth, polarization, cytokine secretion, migration, etc) during cancer progression. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactslownam@purdue.edu  |
| Lucas | Jeffrey | Integrative Neuroscience |
Jeffrey LucasProfessor-Department of Biological Sciences Current Research InterestsMy lab hopes to understand the degree to which complex signals convey "information" among birds. Studies of bird communication, in turn, provide an excellent model for understanding our own language. We have focused most of our work on Carolina chickadees: birds that have one of the most complex vocal systems of any species outside of humans. Indeed, chickadees are one of the few animals known to use syntax in their vocal signals. We have been studying signal complexity in chickadees and the related tufted titmice in the context of the complexity of their social networks. Expanding this work to include an analysis of the hearing capacity of chickadees and other bird species, we were the first lab to show that subcortical processing of sounds varies across seasons. This finding suggests that the neurophysiology of the auditory system changes over the course of the year. Subsequent work has shown fine scale tuning relative to the properties of speciesspecific song. Finally, we are expanding the work on hearing in songbirds to include eagles, with an eye to finding stimuli that can be broadcast from wind turbines with the hopes of reducing mortality rates associated with turbine-bird collisions. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactjlucas@purdue.edu  |
| Luo | Zhao-Qing | Microbiology, Immunology and Infectious Diseases |
Zhao-Qing LuoProfessor of Biological Sciences Current Research InterestsOur laboratory is interested in understanding the mechanisms that allow microbial pathogens to survive and multiply within the hostile host cells and how host cells respond to infection. We use Legionella pneumophila, the causative agent of Legionnaires disease as a model organism. One essential pathogenic factor of this bacterium is the Dot/Icm type IV secretion system that injects approximately 300 virulence proteins (effectors) into host cells to create a niche permissive for bacterial replication. One focus of our research is to determine the function of these proteins and their roles in bacterial infection. The second focus is to examine the mechanism of the detection and response of immune cells to intracellular pathogens, particularly the signaling pathway involved in the detection of the bacterial ribosomal protein RpsL that triggers lysosomal cell death. Finally, we are interested in study the function of Fic proteins found in diverse bacteria. The long term goal of these studies is to elucidate the signal transduction pathways important for bacterial virulence, immune detection and other events important for the establishment of successful infection, such information will be invaluable not only in combating infectious diseases but also in our understanding of cell signaling in both prokaryotic and eukaryotic cells. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contact |
| Lyon | Angeline | Biomolecular Structure and Biophysics, Cancer Biology, Membrane Biology |
Angeline LyonAssociate Professor, Chemistry and Biological Sciences Current Research InterestsWe use biochemistry, biophysics, and cell-based studies to investigate the structure and function of phospholipase C enzymes. These proteins are required for normal cardiovascular function, and are also involved in inflammation and cancer. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactlyonam@purdue.edu  |
| Ma | Jianxin | Plant Biology |
Jianxin MaAssociate Professor Current Research InterestsMy research has been directed toward understanding the nature, timing, mechanisms and biological outcomes of genome evolution in flowering plants, and development and use of high throughput genomics tools for characterization of natural variation that leads to genomic, transcriptomic and phenotypic diversification of plant species. We use a combination of comparative, computational and experimental genomics approaches to analyze the ever-increasing databases of genomic sequences from multiple plant species to address the mechanistic basis that underlies structural and functional genomic changes, with an emphasis on transposable element-mediated gene and genome evolution. We currently have been developing new genomics tools in soybean to facilitate the translation of basic findings into tangible application for germplasm enhancement. Our main research areas include: Comparative, structural and functional genomics of grass and legume species; Translational genomics for crop improvement; Transposable element and genome evolution; Centromere structure and evolution; and DNA recombination, methylation and epigenomics. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmaj@purdue.edu  |
| Mao | Chengde | Biomolecular Structure and Biophysics, Biotechnology, Chemical Biology |
Chengde MaoProfessor, Department of Chemistry Current Research InterestsWe are interested in programmed self-assembly of nucleic acids (DNA and RNA), or DNA nanotechnology. Nucleic acids are information-rich molecules. They have well-defined secondary structures (duplexes) and simple interaction rules (Watson-Crick base pairing). These chemical properties render nucleic acids to be excellent molecules for programmed self-assembly. Since 1982, a wide range of nanostructures have been constructed and find applications in biosensing, imaging, smart drug delivery, vaccine, organizing chemical reactions, plasmonic devices etc. Our current research topics include:
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmao@purdue.edu  |
| Matosevic | Sandro | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Sandro MatosevicAssistant Professor, Industrial and Physical Pharmacy
Current Research InterestsThe Matosevic Lab is focused on cancer immunotherapy for complex solid tumors. Genetically engineering immune cells - specifically, natural killer cells - enables us to reprogram them to more effectively avoid severe functional and metabolic immunosuppression induced by the microenvironment of tumors such as glioblastoma. Through immune cell and gene engineering, the lab focuses on developing new translational immunotherapies based on engineered natural killer cells. We study and develop tools to reprogram, control and direct their therapeutic behavior of and their interaction with the tumor microenvironment. We do so by combining approaches in cell and gene therapy, genetic engineering, immunology, nanotechnology and biomaterials. Projects in our lab are focused on (1) immunoengineering natural killer cells by genetically reprogramming their interaction with and targeting of solid tumors; (2) immunological understanding of the metabolic microenvironment of solid tumors and its suppression of healthy immune function; (3) developing translational tools for adoptive transfer of geneengineered immune cells into patients through cell therapy and cryoprervation. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsandro@purdue.edu
 |
| Mattoo | Seema | Biomolecular Structure and Biophysics, Integrative Neuroscience, Microbiology, Immunology and Infectious Diseases |
Seema MattooAssociate Professor and Chair of PULSe MIID Training Group Current Research InterestsCell signaling and post-translational modifications mediated by Fic proteins. Projects include: 1) Microbial pathogenesis. 2) Parkinson's Disease. 3) Cancer Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsmattoo@purdue.edu  |
| Mendrysa | Susan | Cancer Biology |
Susan MendrysaAssociate Professor of Basic Medical Sciences Current Research InterestsThe unifying theme of the research in the Mendrysa laboratory is the role of the p53 tumor suppressor protein in development, tissue homeostasis, and disease. The regulation of p53 must be tightly controlled as loss of p53 function is a major driver of tumor formation and aberrantly elevated p53 function contributes to the neuro-degeneration associated with human disease including Parkinson’s and Alzheimer’s Disease. A major focus of our current research is to delineating the mechanisms by which p53 function is regulated by the MDM2 protein in cerebellar granule neuron progenitors (GNPs). Disruption of GNP maturation can result in defects in motor coordination and learning, or in medulloblastoma, the most common childhood brain tumor. We primarily use genetically modified mice as a model system in which to place MDM2 and p53 within the molecular pathways that govern the proliferation, differentiation, and cell death of GNPs in cerebellar development and tumorigenesis. Our in vivo genetic studies with mice are complimented by in vitro molecular biology and biochemistry approaches in mammalian cells to delineate the functional and mechanistic relationship of cellular signaling molecules. Using this multi-disciplinary approach, the Mendrysa lab is able to perturb the level of proteins of interest (e.g. p53, MDM2) in the whole animal in order to investigate their physiological roles in normal and pre-cancerous tissues and conduct hypothesis-driven experiments in vitro for follow up analyses. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsmendrys@purdue.edu  |
| Mengiste | Tesfaye | Microbiology, Immunology and Infectious Diseases, Plant Biology |
Tesfaye MengisteAssistant Professor of Plant Pathology Current Research InterestsResearch in Dr. Mengiste's lab focuses on molecular mechanisms of plant responses to economically important fungal pathogens which reduce crop productivity worldwide. Critical genetic components of plant resistance are identified through genetic and genomic approaches in the model plant Arabidopsis, and two crop plants tomato and sorghum. By applying genetic, molecular, and biochemical approaches, his lab seeks to determine how these key components regulate plant immune responses required for resistance. Molecular and biochemical mechanisms of tomato resistance are studied with a focus on the role of tomato receptor like kinases, and their substrates to shed light on tomato immune responses to broad host fungal pathogens. In parallel, attempts are made to translate some of the findings into genetic improvement of crops for disease resistance. In sorghum, the natural variation in the germplasm is being explored to identify genes or genomic regions that confer broad-spectrum resistance to anthracnose and grain mold diseases. The overarching goal is to expedite genetic improvement of sorghum to increase productivity in disease prone sorghum producing regions.
Current research areas
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacttmengist@purdue.edu  |
| Mesecar | Andrew | Biomolecular Structure and Biophysics, Microbiology, Immunology and Infectious Diseases |
Andrew MesecarWalther Professor in Cancer Structural Biology Current Research InterestsThe fundamental research interests of the Mesecar lab involve elucidating the molecular mechanisms and function of therapeutic enzymes and proteins. We wish to understand at the molecular level how enzymes and proteins recognize their substrates, catalyze their requisite chemical reactions, and trigger signal-transduction cascades. Our ultimate goal is to utilize this fundamental scientific knowledge to develop new therapeutics to treat cancer and infectious diseases. To achieve these goals, we integrate a variety of state-of-the-art research tools and approaches including X-ray crystallography, enzyme chemistry and kinetics, molecular biology, bioinformatics, mass spectrometry, and computational chemistry to gain an understanding of the role of protein dynamics and conformational change in molecular recognition and catalysis. We then couple these technologies with high-throughput screening and structure-based design to develop compounds capable of modulating the activity of enzymes and receptors involved in cancer chemoprevention, cancer cell proliferation, cell longevity, and bacterial and viral pathogenesis. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactamesecar@purdue.edu  |
| Metskas | Lauren | Biomolecular Structure and Biophysics, Chemical Biology, Microbiology, Immunology and Infectious Diseases |
Lauren MetskasAssistant Professor Current Research InterestsOur group’s research focuses around functional protein ultrastructures, such as protein lattices involved in pathogen-host interaction. We use a mixture of structural biology, biophysics and chemistry to study these systems and design new experimental methods.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmetskas@purdue.edu  |
| Mickelbart | Michael | Plant Biology |
Michael MickelbartAssociate Professor Current Research Interests• The genetic basis of plant water use • Stomatal development and water use • Use of stable isotopes to study plant processes Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmmickelb@purdue.edu  |
| Mittal | Suresh | Microbiology, Immunology and Infectious Diseases |
Suresh MittalProfessor of Comparative Pathobiology PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contact765-496-2894  |
| Mohammed | Sulma | Cancer Biology |
Sulma MohammedProfessor of Cancer Biology Current Research InterestsOur research focuses on (a) Characterization of an immunocompetent animal model resource for triple-negative breast cancer and investigation of immunopreventative and immunotherapeutic approaches such as vaccines and CAR T therapy; (b) Studying mechanisms of cancer dissemination through the lymphatic system; (c) Studying the biology of triple-negative breast cancer, and (d) Addressing cervical cancer inequality and development of point-of-care-test.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmohammes@purdue.edu  |
| Morgan | John | Biotechnology, Plant Biology |
John MorganProfessor Current Research InterestsMetabolic engineering of photosynthetic organisms Metabolic Flux Analysis Metabolic Modeling Mass transfer of VOCs in flowers. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjamorgan@purdue.edu  |
| Noinaj | Nicholas | Membrane Biology, Microbiology, Immunology and Infectious Diseases, Biomolecular Structure and Biophysics |
Nicholas NoinajAssociate Professor Current Research InterestsMy research interests are in understanding how pathogenic Gram-negative bacteria are able to use virulence factors found on their surface to mediate infection. These virulence factors are found in the outer membrane and belong to a class of surface proteins commonly referred to as outer membrane proteins (OMPs). In particular, my lab will investigate the multi-component complex called the BAM complex, which is responsible for the biogenesis of all OMPs, in hopes of understanding how it is able to fold and insert OMPs into the outer membrane. We will use a combination of techniques to accomplish this including X-ray crystallography, electron microscopy, crosslinking, and various functional assays. Our ultimate goal is to use the information from the structural and functional characterization as a starting point for drug discovery and development targeting the BAM complex in a species specific manner. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactnnoinaj@purdue.edu  |
| Oakley | Chris | Plant Biology |
Chris OakleyEcological and evolutionary genetics: adaptation and adaptive traits (especially freezing tolerance and flowering time); heterosis; plant mating system evolution. Current Research InterestsThe Oakley lab is broadly interested in the ecological and evolutionary genetics of plants. One main focus of our research is the genetic basis of local adaptation. Local genotypes are often found to grow, survive, and/or reproduce better than non-local genotypes, suggesting that adaptation to one environment is costly in other environments (fitness tradeoffs across environments). Despite much empirical study, little is known about the mechanisms and genetic basis of local adaptation. Using locally adapted populations of Arabidopsis thaliana from near the northern and southern edge of the native range, we investigate the genetic basis of local adaptation, adaptive traits (e.g., freezing tolerance and flowering time), and genetic tradeoffs (fitness tradeoffs attributable to individual loci). We have developed a variety of genetic stocks that we use in field and growth chamber experiments in concert with genetic and genomic approaches. A second main focus of our research is the consequences of genetic drift for adaptation and population persistence. A number of factors common in natural populations (e.g., a history of population bottlenecks) can increase both the chance loss of beneficial mutations and the chance fixation of deleterious mutations. Heterosis, the increased fitness in crosses between populations relative to fitness within populations, is thought to be due in part to the masking of these fixed deleterious recessive alleles in the heterozygous state. We are investigating the geographic pattern and genetic basis of heterosis in natural populations of A. thaliana to study the balance between selection and genetic drift in nature. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactoakleyc@purdue.edu  |
| Ogas | Joseph | Plant Biology |
Joseph OgasAssociate Professor of Biochemistry Current Research InterestsWe are interested in characterizing the factors and mechanisms that govern the flexible nature of plant cell identity. To accomplish this goal, we are utilizing a mutant of Arabidopsis thaliana, pickle (pkl), in which the primary root differentiates improperly and expresses embryonic characteristics after germination. Expression of the pickle root phenotype is dependent on gibberellin (GA), a plant growth regulator known to promote such diverse processes as germination, cell elongation, and initiation of flowering. We have cloned PKL, and it codes for a predicted CHD3-chromatin remodeling factor. CHD3 proteins from Xenopus and human cell lines have been shown to associate with histone deacetylase indicating that they may act as negative regulators of transcription. Consistent with such a role for PKL in Arabidopsis, we have shown that LEC1, a promoter of embryonic identity, is derepressed in germinating pkl seedlings. Based on the phenotype of the pkl mutant and the function of proteins that are similar to PKL, our working model is that PKL regulates the transcription of genes in response to GA. Specifically, we propose that PKL establishes transcriptional repression of embryonic genes during germination by altering the structure of chromatin. This model will be tested by the following experimental strategies: examination of the expression of PKL, identification of genes that exhibit PKL-dependent transcription by microarray analysis, analysis of the promoters of those genes, identification of proteins that interact with PKL, and genetic screens for mutations that affect the phenotype of pkl plants. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactogas@purdue.edu  |
| Olson | Matthew | Microbiology, Immunology and Infectious Diseases |
Matthew OlsonAssistant Professor Current Research InterestsThe immune system is comprised of a variety of cell types that work in a highly organized fashion to protect the body from infection and minimize tumor formation. This organizational process is largely carried out by the adaptive immune system with CD4+ T helper (Th) cells acting to trigger inflammation during infection and also suppress unwanted immune responses to non-harmful stimuli (i.e. allergens, food or normal body microflora). The dual nature (i.e. activator and suppressor) of Th cells is possible due to their unique ability to sense the environment and change their function based on these environmental cues. Autoimmune disease occurs when the balance between the inflammatory and regulatory functions of Th cells is disrupted, resulting in excess inflammation that alters physiological processes. Inflammatory bowel disease (IBD) and graft-versus-host disease (GVHD) are two forms of intestinal autoimmune disease and are characterized by the accumulation of highly reactive inflammatory T cells in the intestines. However, the exact mechanisms by which inflammatory Th cells arise during inflammation and cause disease are unclear. In the Olson lab, our goal is to better understand how CD4+ T helper cells drive intestinal inflammation by addressing these key questions:
My laboratory uses a combination of cell and molecular biology approaches to examine signaling pathways associated with T helper cell differentiation. We also utilize pre-clinical models of disease, and high throughput culturing and RNA/protein profiling techniques to identify disease mechanisms and novel mediators of inflammation. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactolson126@purdue.edu  |
| Park | Chiwook | Biomolecular Structure and Biophysics, Computational and Systems Biology |
Chiwook ParkAssociate Professor in MCMP Current Research InterestsProteins are dynamic molecules. Even under native conditions, they do not adopt a single static conformation. Rather, they access many different conformations in their native state ensemble. This native state ensemble includes small fluctuations around the native conformation, partially unfolded forms, and even globally unfolded forms. The distribution of these conformations and the kinetic barriers between the conformational states define the conformational energy landscapes of proteins. My research interest is investigating conformational energy landscapes of proteins and deciphering the relationship between the energetics of proteins and their biochemical functions, such as catalysis, signal transduction, and ligand binding. We use proteolysis as a major tool to probe protein structures and dynamics as well as conventional spectroscopic methods. We also use proteomics extensively for investigating energy landscapes of proteins on a system level. Investigation of conformational energy landscape of proteins on a proteomic scale With the advent of the postgenomic and proteomic era, we face new challenges and new opportunities in protein folding studies. Can we obtain information on the energetics and dynamics of proteins on a proteomic scale? Can we study the relationship between the energetics and function of proteins at a system level? To address these issues, we use proteolysis as a structural probe. Conventional biophysical approaches using spectroscopy and calorimetry allow us to study only one protein at a time. However, by using proteolysis and proteomics tools, we can study energetics of multitude of proteins in a proteome at the same time. Currently, we are attempting to determine global stabilities and unfolding kinetics of proteins on a proteomic scale. This research will allow us to understand why some proteins are more stable than the others and how evolution has shaped the distribution of thermodynamic and kinetic stabilities of proteins in a proteome. Identification of cellular drug targets by proteolysis Energy landscapes of proteins are perturbed by interaction with drug molecules. By monitoring these changes on a proteomic scale using proteolysis, we can identify cellular targets interacting with drug molecules. The cellular targets of effective drugs are often unknown. Moreover, novel chemicals affecting cellular activities are discovered at a tremendous speed by chemical genetics. By knowing the cellular target, we can design better drugs based on the structure and screen other drug candidates in vitro. In addition, these drugs serve as research tools to control cellular functions of the target. Investigation of energetic properties of membrane proteins In spite of functional importance of membrane proteins, conformational energetics of membrane protein structures are not well known yet. Expression and purification of membrane proteins for biophysical studies are still quite challenging. Using proteolysis as a structural tools, we attempt to monitor folding and unfolding of membrane proteins without purifying the proteins. By determining thermodynamic and kinetic stabilities of endogenous membrane proteins in cell lysates, we will investigate the energetic principles governing membrane protein folding. Also, we will determine ligand binding affinities to membrane receptors based on the increase in stability upon ligand binding. Direct investigations of endogeous membrane proteins without purification will allow us to pursue many exciting research opportunities on membrane proteins which have not been possible with conventional methods. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactpark6@purdue.edu  |
| Park | Daniel | Plant Biology |
Daniel ParkPh.D. Current Research InterestsMacroecology, biogeography, biological invasions, phenology, and biodiversity informatics.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdanielpark@purdue.edu  |
| Parkinson | Elizabeth | Cancer Biology, Chemical Biology, Microbiology, Immunology and Infectious Diseases |
Elizabeth ParkinsonAssistant Professor
Current Research InterestsFrom Microbes to Medicines: Cancer is the second leading cause of death in the United States, and antibiotic resistant-bacteria are a growing health crisis. The discovery of novel chemotherapeutics and antibiotics is thus an area of great need. Natural products (NPs) have historically been a bountiful source of anticancer and antibiotic agents. Unfortunately, traditional means of discovering new bioactive NPs are no longer effective due to the problem of rediscovery. Novel strategies to find new bioactive NPs are desperately needed. Research in my group focuses on the discovery of new NPs from cryptic biosynthetic gene clusters (BGCs) and the examination of the anticancer and antibiotic activities of these NPs with a focus on difficult-to-hit targets. The major research areas in my lab are: 1) Chemicals induction of cryptic BGCs for the discovery of novel bioactive NPs 2) Synthesis of peptide macrocycles inspired by cryptic biosynthetic gene clusters 3) High throughput assays for challenging anticancer and antibacterial targets These projects will establish general methods for the discovery of novel anticancer and antibiotic natural products from cryptic biosynthetic gene clusters. In the long term, this approach will allow discovery of many novel anticancer and antibiotic agents and could also be used for the discovery of other types of bioactive molecules. Students in my lab gain experience in organic synthesis, isolation of compounds from complex extracts, natural product structure elucidation, bacterial and mammalian cell culture, high throughput screening, target identification, and bioinformatics. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacteparkins@purdue.edu
 |
| Paschou | Peristera | Computational and Systems Biology, Integrative Neuroscience |
Peristera PaschouProfessor of Biological Sciences Current Research Interests
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactppaschou@purdue.edu  |
| Pienaar | Elsje | Computational and Systems Biology |
Elsje PienaarAssistant Professor Current Research InterestsOur systems pharmacology approach constructs multi-scale, hybrid computational models that describe host immune, pathogen and drug dynamics within an infected patient. In close collaborations with experimentalists, we integrate these computational models with multiple in vitro and in vivo datasets to: predict drug efficacy, optimize treatment, identify new drug targets, and inform future experiments; all in the context of complex host-pathogen-drug interactions. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactepienaar@purdue.edu
 |
| Pluta | Scott | Integrative Neuroscience |
Scott PlutaAssistant Professor Current Research InterestsNavigating our surroundings is a goal-directed process that requires a continual interplay between sensory perception and motor action. To reach the intended destination, orienting towards and acting upon the appropriate environmental cues is essential. The midbrain superior colliculus (SC) is a centralized hub that integrates sensory, motor, and intentional information to guide behavior. Deactivation or lesion of the SC greatly impairs an animal's ability to attend to relevant cues, ignore distractions, and execute the appropriate response. The parallel integration of multiple, disparately organized cortical circuits in the SC is likely critical to forming its rich representations of sensory information that guide its motor decisions. However, the physiological and behavioral significance of this widespread, parallel convergence of cortical input in the SC is poorly understood. The primary focus of my lab is to understand how descending signals from the cerebral cortex influence the activity of neurons in the SC to ultimately shape its intimate connection to behavior. We utilize genetically targeted labeling of cortical neurons and optically manipulate their activity to dissect their physiological and behavioral significance. To form a complete understanding of the sensorimotor loop, we causally test the significance of activity ascending from the SC to higher order networks. Ultimately, research from my lab will provide valuable insight into the neural mechanisms underlying goal-directed behaviors and could enable us to provide better treatment and diagnosis of neurological disorders, such as ADHD, Parkinson’s or schizophrenia. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactLab: LILY B-125
Office: LILY 1-227
Personal Website: https://spluta5.wixsite.com/plutalab
 |
| Porterfield | Marshall | Computational and Systems Biology |
Marshall PorterfieldPULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contact |
| Post | Carol | Biomolecular Structure and Biophysics, Cancer Biology, Computational and Systems Biology, Microbiology, Immunology and Infectious Diseases |
Carol PostProfessor of Medicinal Chemistry and Biological Sciences Current Research InterestsThe research focus of the Post group is broadly described as investigations to understand the regulation and function of protein-protein interactions associated with cell signaling and viruses. Multi-dimensional, multinuclear NMR methods are used to determine 3-dimensional structure of protein complexes. Computational methods are used to study the mechanism of action of antiviral compounds, and the molecular mechanism for phosphotyrosine control of protein function in signal transduction. One research area of our group is the regulation of enzymatic activity and protein-protein association by tyrosine phosphorylation. Such regulation is central to cell signaling, and the transduction of chemical information from outside the cell to control intracellular processes that govern cell growth and differentiation. Protein tyrosine kinases are targets of high interest for cancer therapeutics and for drugs to treat immune diseases. We seek an atomic-level description to begin to understand the molecular basis of regulation. NMR structural studies and computational methods are being used to study how tyrosine phosphorylation controls enzymatic activity of Src-family kinases and other tyrosine kinases in B-cells. One aim is to unravel the molecular mechanism of activation. We are also actively engaged in developing new procedures to facilitate and improve the structure determination method by NMR. Another area of research interest is the study of viral assembly processes using computational biology and high-resolution NMR methods. Results from molecular dynamics on human rhinovirus lead to a new proposal to explain the antiviral activity of WIN compounds against rhinovirus; binding of these compounds in an internal pocket of rhinovirus changes the viral capsid from a rigid-like protein shell to one that is more compressible and thus entropically stabilized. Similar approaches are now being applied to understand virus-receptor interactions and cell entry. High resolution NMR spectroscopy is also used to elucidate the structure and dynamics of alphaviral and retroviral proteins. Specific interactions at a membrane interface between protein core particles and transmembrane glycoproteins necessary for proper budding of alphaviruses are being investigated. In addition, maturation of retroviruses requires specific protein-protein interactions of the gag polyprotein to form infectious particles. NMR structural studies were recently initiated on this system. Methods to obtain solution structures efficiently by a combined use of NMR data and computational tools are under development and should be useful in proteomic initiatives. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcbp@purdue.edu  |
| Powley | Terry | Integrative Neuroscience |
Terry PowleyProfessor-Department of Psychology Current Research InterestsOur laboratory group analyzes the neuroanatomical, physiological, and endocrinological bases of energy homeostasis. Autonomic, visceral, hypothalamic, and hormonal controls of metabolism and feeding are all investigated. Primary emphasis is on the identification and mapping of the neural circuitry controlling feeding and drinking. More generally, we also examine both experimental and theoretical questions pertaining to the issue of brain localization of function. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactpowleyt@purdue.edu  |
| Puthiyaveetil | Sujith | Chromatin and Regulation of Gene Expression, Membrane Biology, Plant Biology |
Sujith PuthiyaveetilAssistant Professor of Biochemistry Current Research InterestsThe regulated synthesis and degradation of proteins produce homeostasis in the functional proteome of the cell (proteostasis). Chloroplasts are subcellular compartments in which photosynthesis, the conversion of light energy into chemical energy, takes place in plants and algae. Plant chloroplasts spend nearly 70% of the ATP budget set aside for synthesis and degradation of cellular proteins. This staggering energetic cost of plastid proteostasis arises from the high abundance and turnover of photosynthetic proteins, as dictated by the need to harvest a dilute and dangerous source of energy – sunlight. Strategies that ensure economical protein synthesis in plastids are therefore of outmost importance to photosynthetic efficiency, the energy balance sheet of the cell, and plant health. However, relatively little is known about the contribution of regulated protein synthesis in plastid proteostasis. We investigate the role of a bacterial-type gene regulatory protein termed Chloroplast Sensor Kinase (CSK) in plastid proteostasis. Our recent research shows that CSK employs an iron-sulfur cluster to perceive and propagate the photosynthetic electron transport signal to plastid gene expression machinery. We use system biology approaches in the model plant Arabidopsis to test the proposed plastid proteostasis function of CSK. Photosystem II (PS II) carries out the formidable water-splitting reaction in photosynthesis and thereby extracts electrons and protons necessary for the anabolic reactions of life. Incomplete water-splitting and or inadvertent electron transfer to molecular oxygen generate highly reactive oxygen free radicals that damage a key protein subunit in PS II. PS II repair cycle is a highly orchestrated molecular process wherein the damaged PS II subunit is selectively degraded and replaced with a newly synthesized copy. Using biophysical and biochemical approaches we investigative the role of post-translational modifications in the repair cycle of PS II. Diatoms are a group of mostly aquatic photosynthetic algae with a plastid of red algal origin. These prolific photosynthesizers, while constituting just a fraction of the total biomass on earth, fix as much carbon as all rainforests combined. We use the model diatom Phaeodactylum tricornutum to decipher the genetic basis of diatoms’ high photosynthetic light use efficiency. Our recent research finds evidence for regulation of plastid genes in diatoms. In Phaeodactylum the expression of multiple plastid genes seem to be under the control of the redox state of plastoquinone (PQ), an electron carrier that links the two photosystems. We currently study the physiological significance of PQ-redox responsive plastid gene expression and its underlying regulatory circuitry in diatoms. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactspveetil@purdue.edu  |
| Ratliff | Timothy | Microbiology, Immunology and Infectious Diseases, Cancer Biology |
Timothy RatliffDistinguished Professor Department of Comparative Pathobiology and the Robert Wallace Miller Director of the Purdue Cancer Center Current Research InterestsDr. Ratliff’s laboratory focuses on understanding immune regulation and the development of alternative approaches to treating urologic cancers, primarily bladder and prostate cancers, through the modulation of anti-cancer immunity. Current studies focus on prostate inflammation, its immune regulation and its impact on prostate stem cells, gene expression in prostate tissue, and cancer development. The intent is to develop a better understanding of the inflammatory factors contributing to cancer development and to use the information gained to develop novel approaches to treating prostate cancer through modulation of the immune response. Genetically modified mouse models are used to probe inflammation, immune regulation, the development of autoimmunity and anticancer effector mechanisms. Regulatory cells termed myeloid-derived suppressor cells have been linked to regulation of prostate inflammation and are observed early in genetically modified mouse spontaneous prostate tumor models. Determining how to control myeloid-derived suppressor cell function is a focus that is anticipated to provide a new approach to augmenting antitumor immunity. Likewise, understanding the impact of inflammation on prostate stem cells is anticipated to advance knowledge about the mechanisms of prostate growth and cancer development. Studies to understand aspects of cholesterol metabolism on prostate cancer growth are an active component of our research activities. Cholesterol sulfate is an important metabolite of cholesterol in prostate cancer. Cholesterol is converted to cholesterol sulfate by the sulfotransferase enzyme, SULT2B1b. We observe the induction of apoptosis if SULT2B1b is eliminated from prostate cancer cells. The apoptotic cascade appears to be initiated through the androgen receptor. Current studies are focused on understanding the mechanisms by which elimination of SULT2B1b initiates apoptosis. Studies to improve the treatment of bladder cancer through the development of nano-delivery approaches for intravesical therapy are in progress. The fibronectin binding protein, previously identified as an important attachment protein for mycobacteria, was observed to mediate antitumor activity in a bladder tumor model and alter the bladder inflammatory process. The fibronectin binding protein has the capacity to deliver nanoparticles to bladder cancer cells is being tested as a new approach for delivery of therapeutic agents for the treatment of superficial bladder cancer. The approach has the potential to not only treat superficial bladder cancer but also to treat a bladder inflammatory condition termed interstitial cystitis. More basic studies focus on the impact of platelet-derived immunomodulatory molecules on the adaptive immune response. Precursor frequency of antigen specific T and B lymphocytes is very low prior to antigenic recognition and clonal expansion of the reactive cells. Control of pathogenic microbes early in the infection period is linked to innate immunity prior to clonal expansion of antigen specific adaptive immunity. Studies in the Ratliff laboratory implicate platelet-derived ligands as mediators of the early amplification of the adaptive immune compartment. Ratliff’s team hypothesizes that platelet-derived ligands are the earliest signals to the adaptive immune compartment that enables rapid and ultimately optimal expansion of the adaptive immune response to the invading microbe. Studies are in progress to fully characterize the role of platelets in the modulation of adaptive immunity. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacttlratlif@purdue.edu  |
| Reppert | Mike | Biomolecular Structure and Biophysics, Computational and Systems Biology, Plant Biology |
Mike ReppertAssistant Professor Current Research InterestsOur primary research interest is to understand the "structure-spectrum" relationship in photosynthetic chlorophyll proteins. By constructing point mutants in both model proteins and in intact cyanobacterial photosystems (especially photosystem II), we are working to explore the role of electrostatic, steric, and ligation interactions in the structure-based tuning of chlorophyll properties. Simultaneously, we are using these experimental insights to build advanced simulation models and user-friendly, online software applications (see https://nanohub.org/tools/pigmenthunter) that can be used to rationally design modified chlorophyll proteins for efficient biofuel production. Finally, we are also working to develop novel approaches to using vibrational spectroscopy as a residue-specific probes of protein structure in complex, disordered systems.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactreppertm@purdue.edu  |
| Rispoli | Joseph | Biomolecular Structure and Biophysics, Biotechnology, Integrative Neuroscience |
Joseph RispoliAssistant Professor - Weldon School of Biomedical Engineering Current Research InterestsDr. Rispoli’s research incorporates novel methodology and instrumentation for magnetic resonance imaging and spectroscopy. He is particularly interested in anatomically-tailored radiofrequency coil design and in vivo spectroscopy, for research applications in neuroscience and breast cancer. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjrispoli@purdue.edu  |
| Robinson | J Paul | Biotechnology |
J Paul RobinsonProfessor of Cytomics Current Research InterestsWe present cytometry and confocal microscopy education and research material, maintain the cytometry email archive,and links to cytometry web sites and suppliers worldwide. Our goal is to share high-quality information and to maintain electronic crossroad for cytometry. In 1993, we established the first web site in cytometry. It has matured and evolved with time. We did this because of our interest and dedication to the philosophy of sharing educational material. Visit our web site at: http://www.cyto.purdue.edu/ PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactwombat@purdue.edu  |
| Robles | Estuardo | Integrative Neuroscience |
Estuardo RoblesProfessor of Biological Sciences Current Research InterestsResearch in my laboratory is focused on understanding how the vertebrate nervous system processes visual information to generate appropriate behaviors. We study visually-responsive neurons in the larval zebrafish, a vertebrate animal model that is ideal for 1) targeted manipulation of genetically-defined neurons, 2) in vivo imaging of neuronal structure and function, and 3) quantitative analysis of visually-evoked behaviors. A major current focus of the lab is to functionally and genetically characterize retinal ganglion cells (RGCs), the neurons that transmit visual information from the retina to the brain. Future studies will be aimed at defining the structural and functional properties of visual neurons in the brain, with the ultimate goal of discovering fundamental principles underlying the organization of visual circuits. The experimental tools that we use include genetic methods to label specific neuron types, confocal microscopy to analyze neuronal morphology, and multiphoton microscopy to monitor visually-evoked neuronal activity.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactroblese@purdue.edu  |
| Rochet | Jean-Christophe | Biomolecular Structure and Biophysics, Chemical Biology, Integrative Neuroscience |
Jean-Christophe RochetProfessor of Med Chem Mol Pharm; John and Donna Krenicke Director, PIIN Current Research InterestsResearch in my laboratory is aimed at understanding the role of protein aggregation in neurodegenerative disorders. Generally, proteins adopt a precise three-dimensional structure that determines their function. However, mutant polypeptides or proteins subjected to environmental stress are often incorrectly folded. At high concentrations, proteins that lack a compact fold tend to undergo spurious interactions to form abnormal, high-molecular-weight complexes. This phenomenon, referred to as protein "aggregation" or "misassembly," is associated with various human diseases. We have chosen to address the role of protein misassembly in agre-related neurodegenerative disorders, including Parkinson's disease (PD), dementia with Lewy bodies (DLB), and Alzheimer's disease. Our research objectives are as follows: (i) Identify molecular mechanisms underlying protein misassembly in PD and AD. (ii) Determine how post-translational modifications affect protein aggregation in PD and AD. (iii) Identify cellular pathways perturbed by protein aggregates. (iv) Elucidate mechanisms underlying the prion-like spreading of protein aggregates throughout the brain. (v) Develop therapeutic strategies to alleviate pathology and neurodegeneration.(vi) Develop new methods and models to investigate protein aggregation. These questions are addressed using an interdisciplinary approach, including biochemical and biophysical analyses of recombinant proteins and the development of cellular models (e.g. primary rodent neuronal cultures, human iPSC-derived neurons) as well as in vivo models (e.g. rats injected in the brain with viral vectors or preformed amyloid-like fibrils). Finally, an important goal is to identify chemical entities (e.g. small molecules, peptidomimetics) to study and ultimately treat these devastating illnesses. Our research benefits from collaborations with experts in engineering, data science, and human medicine in the rich scientific environment provided by the Purdue Institute for Integrative Neuroscience. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjrochet@purdue.edu  |
| Sanders | David | Microbiology, Immunology and Infectious Diseases, Biomolecular Structure and Biophysics |
David SandersAssociate Professor of Biological Sciences Current Research InterestsRetroviruses, through the process of pseudotyping, can acquire the glycoproteins of certain other enveloped viruses and can utilize them for entry into cells. We have demonstrated, for example, that the Ebola virus glycoprotein can be incorporated into replication-defective retroviruses and that it can mediate viral entry into cells. Our experiments allow us to study the entry of viruses such as the Ebola and Marburg viruses in a fashion that is independent of the other steps in the viral life cycle and to do so in a safe and quantitative manner. Our pseudotyped viruses may also have applications in gene-transfer and gene-therapy experiments. Using the system that we have developed, we have found, for example, that the biochemistry of Ebola virus entry resembles that of bird retroviruses. Our research supports the hypothesis that these viruses shared a common ancestor. This indicates that it is likely that the Ebola virus either currently has a bird as its natural host or that it has evolved from a bird virus. Our studies have also allowed us to determine the disulfide-bond map of the Ebola glycoprotein and to propose that reduction of the disulfide bond between the two subunits of the Ebola glycoprotein complex, GP1 and GP2, is a critical step in the entry of Ebola virus into cells. Furthermore, we have shown that removal of a region of O-glycosylation of the protein enhances processing and its incorporation into recombinant pseudotyped retroviruses. This modification allows for a greatly improved efficiency of gene transfer by the recombinant viruses. Our recombinant viruses bearing the Ebola virus glycoproteins are particularly suited for gene therapy for diseases such as cystic fibrosis. We have invented viruses that have the shells of alphaviruses and the cores of retroviruses. These novel pseudotyped viruses have numerous advantages as gene delivery/gene therapy agents. We have recently demonstrated in a collaboration with researchers at the University of Iowa that these viruses have superior capacity for introducing genes into the liver and brain glial cells in vivo. They therefore possess great promise for the treatment of a number of diseases. We and our collaborators have identified the cysteine residues that participate in the murine leukemia virus envelope-protein intersubunit cystine bridge. Analysis of the sequence surrounding the disulfide-forming residues suggests that a thiol-disulfide exchange reaction plays a critical role in the activation of membrane fusion. Our hypothesis is that thiol-disulfide exchange reactions are fundamental to solving the basic problem of virus structure and entry, i.e., how to be stable outside a cell and yet be able to disassemble during penetration. We have also examined how an organism can evolve to acquire resistance to retrovirus-mediated disease and have shown that this can be achieved through the acquisition of a fusion-defective envelope protein. Our findings suggest an approach to the design of a therapy for the prevention or treatment of AIDS. In another aspect our research we have determined the three-dimensional structure of the Methanosarcina thermophila acetate kinase in collaboration with the laboratories of Greg Ferry at Penn State and Miriam Hasson at Purdue University. As we had predicted, despite the absence of identity between the sequences of acetate kinases and proteins of previously known three-dimensional structure, the core fold of acetate kinase is the same as that of the glycerol kinase/hexokinase/actin/hsp70 superfamily of phosphotransferases. The superfamily is now known as the ASKHA (Acetate and Sugar Kinases/Hsp70/Actin) phosphotransferases. Biochemical, evolutionary, and geochemical considerations support the proposal that an acetate kinase may be the ancestral phosphotransferase of this very ancient and diverse superfamily. We are continuing our studies investigating the enzymology of acetate kinase and the three-dimensional structures of isobutyrate kinase and exopolyphosphatases, which are also members of the ASKHA phosphotransferase superfamily. Our recent findings provide new insights into the extraordinary processiveness of the bacterial exopolyphosphatases and the mechanism by which bacteria respond to starvation conditions. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactretrovir@purdue.edu  |
| Sangha | Susan | Integrative Neuroscience |
Susan SanghaAssistant Professor, Behavioral Neuroscience Current Research InterestsMy research program focuses broadly on the mechanisms underlying fear suppression, using a combination of behavioral, electrophysiological, and viral DREADDbased techniques in the freely behaving rat. While the rat is learning about fear, safety and reward signals, we track biological changes in the brain as the animal is learning in order to map out the neural circuits necessary for accurate discrimination of fear, safety and reward signals. For more information, visit www.sanghalab.com PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactsangha@purdue.edu  |
| Scarpelli | Matthew | Biotechnology, Cancer Biology, Microbiology, Immunology and Infectious Diseases |
Matthew ScarpelliAssistant Professor Current Research InterestsArea 1) Investigating the role of the immune system in the biological response to radiation. There is increasing evidence that radiotherapy stimulates the immune system through the release of tumor antigens from dead tumor cells. These antigens are recognized by the immune system, stimulating an immune response against residual tumor cells and in rare cases distant metastases through the abscopal effect. However, these responses are not fully understood and distant metastatic responses to localized radiotherapy are exceedingly rare. Investigating this phenomenon and leveraging it to improve the efficacy of radiotherapy is a guiding principle of the lab. Area 2) Establishing imaging biomarkers of the immune system. There is no established clinical method for measuring immune responses in tumors or specific organs. Due to the dynamic nature of the immune system, conventional tissue biomarkers derived at a single timepoint (e.g., tissue biopsies), generally do not provide reliable measures of the immune system. Furthermore, conventional imaging methods, often used to track changes in tumor size, are insensitive to the immune system and its effects on the tumor. Dr. Matthew Scarpelli’s lab prioritizes development of molecular PET and MR imaging techniques that are sensitive to these effects and could be used as subjective measurements of immune response. Area 3) Development of image-guided radio and immunotherapies. Dr. Scarpelli’s laboratory seeks to expand image-guidance in radiotherapy to include immunogenic targeting and adaptive therapy. This is accomplished through translational studies and collaborations with various medical centers. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmscarpel@purdue.edu  |
| Schaser | Allison | Integrative Neuroscience |
Allison SchaserAssistant Professor in the Department of Speech, Language, and Hearing Sciences Current Research InterestsAllison Schaser, Ph.D., CCC-SLP is an Assistant Professor in the Department of Speech, Language, and Hearing Sciences at Purdue University. She holds a bachelor’s degree in Speech-Language Pathology from Miami University in Ohio and a master’s degree in Communication Sciences and Disorders from the University of Wisconsin-Madison. She earned her Ph.D. in Communication Sciences and Disorders from the University of Wisconsin-Madison in 2015 and recently completed a post-doctoral fellowship in the Neurology department at Oregon Health & Science University focused on understanding the pathophysiology of age-related synucleinopathies like Parkinson disease (PD) and Dementia with Lewy Bodies (DLB). In her research, she uses advanced imaging tools and an alpha-synuclein fibril seeding approach to study protein aggregation in a mouse model of PD and DLB. Her lab specializes in merging clinical issues, animal behavior, and exploration of pathology in the cranial sensorimotor system to characterize and treat voice, communication, and swallowing deficits in age-related synucleinopathies. Allison was president of the Portland-based non-profit Women in Science Portland while she completed her postdoctoral fellowship and is passionate about continuing her work building supportive networks to promote retention and career development opportunities for women in the sciences at Purdue. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactaschaser@purdue.edu  |
| Searle | Catherine | Microbiology, Immunology and Infectious Diseases |
Catherine SearleAssistant Professor Current Research InterestsResearch in the Searle lab investigates the ecology of infectious diseases in freshwater systems. We are particularly interested in how changes to the abiotic and biotic environment affect disease risk in individuals, populations, and communities. To understand these processes, we use a combination of field surveys, experiments (lab and mesocosm) and modeling. Our primary hosts systems are freshwater zooplankton (Daphnia) and amphibians (with a focus on chytridiomycosis). These complementary systems allow us to investigate a wide range of ecological questions and mechanisms. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsearlec@purdue.edu  |
| Sepulveda | Maria | Computational and Systems Biology, Integrative Neuroscience |
Maria SepulvedaProfessor Current Research InterestsNeurotoxicity and thyroid toxicity of per- and polyfluoroalkyl substances (PFAS) on Xenopus and leopard frogs. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmssepulv@purdue.edu  |
| Shah | Kavita | Cancer Biology, Chemical Biology, Integrative Neuroscience |
Kavita ShahWalther Professor Current Research InterestsOur research goal is to develop and apply chemical tools to dissect biological pathways. We specifically focus on Protein Kinase and G Protein superfamilies. Protein Kinases are a large family of signaling proteins (538 kinases have been discovered in human genome). Deregulated kinase signaling is implicated in more than 400 diseases including cancer, neurological diseases, metabolic diseases, rheumatoid arthritis, cardiovascular diseases and psoriasis. Similarly, Guanine nucleotide binding proteins (G Proteins) constitute another large family of signaling proteins (220 members) that are finely interwoven in every aspect of cellular signaling. Ras, the founding member of this family, is implicated in more than 30-40% of all known human cancers. Our research group is deeply interested in unraveling the molecular mechanisms of cancer and neurodegeneration in cells, animal models and human diseased tissues, with the goal of identifying novel therapeutic targets, which can be targeted independently or in combination to prevent or treat human diseases. To this end, we employ a combination of chemical, genetic, genomic and proteomic approaches. Our research activities generally fall into three categories: (1) Exploring the mechanism of neurodegeneration in Alzheimer’s disease for developing mechanism-based therapies. (2) Identification and Validation of Novel Therapeutic Targets in cancer, particularly those proteins which are cancer-specific. (3) Development of Chemical Tools for dissecting signaling pathways in cells. We are also involved in the synthesis and screening of small molecules which can potentially act as anti-cancer agents directed towards specific targets in cells. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactshah23@purdue.edu  |
| Shannahan | Jonathan | Biotechnology |
Jonathan ShannahanAssociate Professor of Toxicology
Current Research InterestsThe primary focus of my research group is the mechanistic investigation of nanomaterial-biological interactions and their influence on the applications of nanotechnology. Current research projects include 1) nanomaterial-biomolecule interactions in susceptible subpopulations existing with underlying disease states, 2) immune responses to metal and emerging two-dimensional nanomaterials, 3) cardiopulmonary and immune toxicity assessment of environmental and occupational nanomaterial exposures. Overall the goal of our research is to understand nanomaterial biological interactions and responses in order to maximize the potential benefits of nanotechnology while insuring that possible adverse health effects are avoided. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjshannah@purdue.edu  |
| Shi | Riyi | Integrative Neuroscience |
Riyi ShiProfessor of Neuroscience and Biomedical Engineering Current Research InterestsMy laboratory is interested in the cellular and molecular underlying mechanism of nerve damage and recovery. We are particularly interested in the responses of the mammalian spinal cord following mechanical injury. We employ a noval double sucrose-gap chamber developed in this laboratory, for physiological recording and subsequently histological analysis of isolated guinea pig spinal cord. We have made progress in both understanding the nature of the injury as well as producing new potential treatments. For instance, we have been investigating the effect of 4-aminopyridine, a potassium channel blocker, in enhancing action potential conduction in chronic and acutely injured mammalian spinal cord. The search for other drugs with potential therapeutically value is also being conducted. Such studies provide strong support for the effective use of certain drugs (like 4-AP and methylprenisolone) in treating clinical spinal cord injury in man. We are also interested in the effects of physiological variables, such as ionic concentrations, O2 tension, temperature and pH in controlling membrane sealing after axonal damage. Sealing is a critical step in the recovery process following spinal cord injury. More recently, collaborating with Dr. Borgens, a new area of research has been identified. We have successfully reconnected two completely severed guinea pig spinal cord segments using a molecular surfactant (polyethylene glycol, or PEG). We have also shown that PEG can enhance the recovery of spinal cord following compression injury, a more clinically relevant type of injury. Currently, in vivo as well as in vitro investigations are underway to explore the clinical usage of PEG. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactshir@purdue.edu  |
| Simpson | Garth | Membrane Biology |
Garth SimpsonAssisant Professor — Analytical Chemistry Current Research InterestsThe emphasis of the Simpson Group is on the development of novel techniques and their application in addressing important problems at biological interfaces. As biotechnology continues to be an area of rapid growth both academically and economically, so grows the need for new tools to interrogate biological systems. Nonlinear Optics of Biological Interfaces The growing availability of intense, pulsed laser sources has launched a new age in nonlinear optical spectroscopy. The simplest nonlinear optical phenomenon is the frequency doubling of light (second harmonic generation or SHG). SHG is forbidden in random media but can arise from surfaces, where symmetry breaking at the interface generates a localized surface-specific signal. Because of this surface specificity, SHG allows unique opportunities in studies of buried interfaces, where other measurements are often dominated by the bulk response. Furthermore, the basic measurements themselves are remarkably simple to perform and typically yield readily detectable signals from surface films less than a single molecular layer thick. A primary focus of our recent research has been the development of methods to mine the exquisite structural information contained within the polarization dependence of the nonlinear signals. Studies span the range from development of new fundamental theories regarding the nature of nonlinear optics to construction of state-of-the-art instrumentation to measurements of structure and function in biological membranes and protein films. Nanoscale AC Electrokinetics Particles and cells behave strangely in alternating current (AC) electric fields. Particles can be attracted or repulsed from sharp electrode tips (dielectrophoresis), can be made to rotate (electrorotation), and can link together to form long chains (pearl-chaining). AC fields can induce nanoscopic pores in cell and vesicle membranes, allowing the introduction of macromolecules (electroporation). Additionally, cells and vesicles can be made to fuse together (electrofusion). We are developing a number of methods to allow extension of these remarkable phenomena to nanoscopic measurements and systems. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactgsimpson@purdue.edu  |
| Sintim | Herman | Chemical Biology, Microbiology, Immunology and Infectious Diseases |
Herman SintimProfessor of Chemistry Current Research Interests1) Cyclic dinucleotide signaling in bacteria and immune cells: we use chemical probes, as well as global proteomics, to identify signaling pathways and key proteins that are involved in cyclic dinucleotide (Cdn) signaling. Inhibitors of Cdn signaling have the potential to be used as anti-cancer and anti-inflammatory agents. 2) Bacterial quorum sensing: Bacteria communicate with each other via small diffusible signal molecules (autoinducers). We are interested in developing novel inhibitors of quorum sensing as anti-virulence and anti-biofilm agents. 3) Inhibitors of protein kinases (examples are: FLT3, ABL1, ROCK1/2, LRRK2, RET, CDKs): There are over 500 protein kinases in mammalian cells and these are involved in diverse processes. Protein kinases are targets for several disease states (such as cancer, Parkinson's, Alzheimer's and inflammatory diseases). Many cancers are driven by mutated kinases. We are developing orally bioactive protein kinase inhibitors against FLT3 (AML), ABL1(CML), RET(NSCLC), LRRK2 (potentially Parkinson's) and ROCK1/2 (metastatic cancer, glaucoma and stroke). 4) Novel antibacterial and anti-biofilm agents: We are developing novel compounds that are active against methicillin-resistant Staphylococcus aureus, MRSA. We are particularly interested in small molecules (MW<500) that are able to clear MRSA from host cells, such as macrophages. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact |
| Sjogren | Benita | Integrative Neuroscience, Membrane Biology, Chemical Biology |
Benita SjogrenAssistant Professor, Medicinal Chemistry and Molecular Pharmacology Current Research InterestsG protein-coupled receptors (GPCRs) represent the largest family of receptors and are important drug targets with 40-60% of currently FDA approved drugs targeting the receptors themselves or downstream mechanisms. One important regulatory mechanism of G protein signaling is mediated through a family of GTPase accelerating proteins, Regulator of G protein Signaling (RGS) proteins. RGS proteins modulate GPCR signaling by binding to and accelerating GTP hydrolysis on active Gα subunits of heterotrimeric G proteins. This results in attenuation of duration and amplitude of GPCR signaling. The majority of the more than 20 RGS proteins identified to date also possess additional functions not related to their canonical effect on G proteins. In the past 20 years, RGS proteins have emerged as novel drug targets in numerous disease states, such as hypertension, cancer and Parkinson disease among others. RGS proteins are tightly regulated through transcriptional, epigenetic and posttranslational mechanisms, such as degradation through the ubiquitin-proteasomal pathway. Furthermore, expression of RGS proteins is often altered during pathogenesis, causing dysregulation in GPCR signaling, that can cause or worsen the disease. Thus, the work in our lab is focused on two central questions; 1) To understand the mechanisms by which levels and function of RGS proteins are regulated and; 2) How the knowledge of these mechanisms can be applied in drug discovery. Our current projects cover RGS2, RGS10, and the three members of the RZ family (RGS17, 19 and 20). RGS2 is rapidly degraded through the ubiquitin-proteasomal pathway. In this essential system, a protein is targeted for degradation through the enzymatic coupling of a chain of ubiquitin molecules that serves as a recognition signal for the proteasome. Over 600 E3 ligases serve as substrate-recognizing components with great selectivity towards different proteins. Through high-throughput siRNA screening we identified a novel multiprotein E3 ligase responsible for RGS2 protein degradation. Current work aims to dissect the precise molecular mechanisms and interactions within this complex to determine what drives RGS2 protein degradation, using biochemical, structural and cell based systems. Another angle of this project is to develop a high-throughput assay to screen for novel inhibitors of RGS2 targeting by this E3 ligase. We are also studying the role of posttranslational modulation of RGS2 in diseases such as cardiovascular disease, asthma, neurodegeneration and uveal melanoma. RGS10 is heavily regulated through epigenetic mechanisms and has been implicated as a key regulator of neuroinflammation. This project aims to develop a high-throughput screening assay to be used for small molecule and siRNA screening for novel modulators of RGS10 in microglia. Through this work we aim to not only identify molecules that could be developed into novel therapeutics, but also unveil mechanism that regulate RGS10 expression in microglia. For the RZ family of RGS proteins we are investigating the role that palmitoylation plays in subcellular localization, protein stability and activity of these proteins. This is an early stage project, but it could have important implications in how these proteins regulate breast cancer progression. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact765-494-3501  |
| Specht | Aaron | Chemical Biology, Biotechnology |
Aaron SpechtAssistant Professor Current Research InterestsAaron Specht is an Assistant Professor in the School of Health Sciences. He received his B.S. from Purdue in Honors Physics and Ph.D. from Purdue in Medical and Health Physics. He completed postdoctoral training at the Harvard T.H. Chan School of Public Health where he remains active as part of their Trace Metals Laboratory utilizing his x-ray fluorescence devices. His research focuses on the development, application, and understanding of exposure assessment for elemental and radiation exposures in environmental and occupational health studies. He has developed instrumentation capable of in-field or in-lab measures that utilize a non-destructive, non-invasive, and cost-effective elemental quantification method using x-ray fluorescence. This includes everything from measuring nail, bone, or blood to determine exposure to a human or in animal studies to measuring soil, ash, or air filters to determine ongoing exposures at particular sites. He has many interesting collaborative projects ranging from typical studies in hospital settings around the globe to measuring condors on the cliffs of the Grand Canyon. Feel free to email him with any questions, lab opportunities, or collaborative efforts.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactaspecht@purdue.edu  |
| Stahelin | Robert | Membrane Biology, Microbiology, Immunology and Infectious Diseases, Biomolecular Structure and Biophysics |
Robert StahelinRetter Professor of Pharmacy Current Research InterestsThe main focus of the lab is mechanisms by which lipid-enveloped viruses (coronaviruses, filoviruses and paramyxoviruses) replicate via assembly and budding in human cells to form new virus particles. The lab uses an array of biochemical and biophysical techniques (including structural biology) in vitro and in cells to come to quantitative and confident conclusions in mechanisms by which viral proteins hijack host cell lipids and proteins for their replication cycle. Interdisciplinary research focused on biological membranes has revealed them as signaling and trafficking platforms for processes fundamental to life. Biomembranes harbor receptors, ion channels, lipid domains, lipid signals, and scaffolding complexes, which function to maintain cellular growth, metabolism, and homeostasis. Moreover, abnormalities in lipid metabolism attributed to genetic changes among other causes are often associated with diseases such as cancer, arthritis and diabetes. Pathogens, including viruses, and bacteria, often hijack human components of lipid metabolism and replicate using biological membranes as platforms or utilize lipids to produce energy during the replication cycle. Thus, there is a need to comprehensively understand molecular events occurring within and on membranes as a means of grasping disease etiology and identifying viable targets for drug development.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactrstaheli@purdue.edu  |
| Staiger | Chris | Membrane Biology, Plant Biology |
Chris StaigerDistinguished Professor of Biological Sciences, and Current Research InterestsThe microtubule and microfilament cytoskeleton is essential for many dynamic cellular process including: cell division, cytoplasmic streaming, cellular architecture and morphogenesis. The ultimate goal for our research is to analyze the regulatory and structural molecules associated with these cytoskeletal elements and to discover how these contribute to plant development. We have developed two complementary strategies for identifying and characterizing the components necessary for cell division and morphogenesis. The first, a cytological and molecular genetic analysis of cell division, makes use of a large collection of maize meiotic mutants. The second approach is to isolate actin-associated proteins using molecular and biochemical techniques, to characterize their function in vitro, and to use these as probes to dissect cytoskeletal function in living plant cells. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactstaiger@purdue.edu  |
| Stauffacher | Cynthia | Membrane Biology, Cancer Biology, Biomolecular Structure and Biophysics |
Cynthia StauffacherProfessor of Biological Sciences Current Research InterestsHigh resolution structural studies combined with techniques of molecular biology are beginning to reveal the workings of biological molecules at the molecular level. My research interest is in using X-ray crystallography to study the details of large macromolecular systems where a set of proteins work together to perform a biological function. Membrane proteins are often organized into these large systems and are of particular interest, as very few membrane protein structures are known. One system we study is the family of ABC-transporters, ubiquitous membrane protein transport systems which include such medically important molecules as the multidrug resistance P-glycoprotein. We have solved the 1.6Å structure of the ATP binding cassette (RbsA) of the E. coli ribose transporter and the 3.2Å structure of the repressor protein (RbsR) which controls transcription of the rbs operon. Models of the mammalian proteins have been built which suggest how interdomain communication occurs. We are continuing to investigate the mechanism of trans-membrane transport by the RbsA/C complex, studying structures of substrate analog and inhibitor complexes. Membrane associated enzymes also perform important biological functions, such as mammalian HMG-CoA reductase, which catalyzes the first committed step in the synthesis of cholesterol. The structure of a bacterial analogue HMG-CoA reductase has been solved at 2.2Å resolution. Difference Fourier studies have revealed the substrate binding sites, as well as a site for anti-cholesterol drug binding. Since this class of bacterial HMG-CoA reductases are found in many common pathogens, we have begun to investigate these enzymes as antibacterial drug targets. Signal transduction in many systems is controlled by a highly regulated cascade of phosphorylation and dephosphorylation steps which begin at the membrane surface. We have solved the structures of three low molecular weight tyrosine phosphatases at high (1.6-2.2Å) resolution. We have identified the active site loop, found regions determining enzyme specificity and have investigated substrate, activator and inhibitor complexes which allow us to understand the biological mechanism at the molecular level. Additional studies in molecular detail on membrane-active toxins, such as colicin E1 and its BtuB receptor target, as well as the staphylococcal enterotoxins, which target immune cell surface molecules, will allow us to more fully understand the complex biochemistry that occurs at the membrane surface. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcstauffa@purdue.edu  |
| Strickland | Elizabeth | Integrative Neuroscience |
Elizabeth StricklandProfessor-Department of Audiology and Speech Sciences Current Research InterestsI have a background in psychology, audiology and neuroscience. In my research I have people listen to carefully designed tones and noises, and from their responses I come up with theories about how the auditory system works. I use stimuli that are very precisely controlled in time and in frequency, such as tones and noises, because the auditory system is so complex that it is only by using very controlled stimuli that we can get a clear picture of what is happening. Over the past few years I have been working on a very exciting line of research examining how the auditory system changes as it is stimulated by sound. One example of this sort of change is the fact that a brief tone that occurs at the onset of a longer duration noise is harder to hear than one that is delayed from the onset of the noise. This effect, often called overshoot, has been attributed to effects as peripheral as adaptation in the VIIIth nerve, and as central as attention. In my research, I have found strong evidence that overshoot actually results from the auditory system turning down amplification in the cochlea as it is stimulated by sound. This is a critically important finding, because it explains a wealth of other very basic psychophysical data in addition to the overshoot effect. Also, it has been known for some time that there is efferent feedback to the cochlea, which has been shown to turn down cochlear amplification in anesthetized animals, but it has been difficult to demonstrate its effects behaviorally. The results of my research in awake, behaving humans suggest that the efferent system plays a key role in auditory perception. This also has important clinical applications. In the past, we have tended to compare normal and impaired hearing as if the auditory system were static. My research suggests that the normal auditory system is changing in a dynamic manner in response to sound, while the impaired system does not, and this could explain why hearing impairment causes more difficulty than might be expected from a simple elevation in hearing threshold. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactestrick@purdue.edu  |
| Suter | Daniel | Integrative Neuroscience, Membrane Biology |
Daniel SuterProfessor Current Research InterestsOur laboratory focuses on the mechanisms of neuronal growth cone motility and guidance during development and regeneration. We use two model systems, Aplysia and zebrafish. Using the large Aplysia neurons in culture, we investigate how the growth cone integrates its sensor, signaling, and motility functions to achieve directional movements towards target cells and establish functional connections. Specifically, we would like to understand how extracellular signals, such as from cell adhesion molecules, are transduced inside the cell to affect the underlying cytoskeleton. Our current studies aim at the understanding of reactive oxygen species (ROS) signaling and mechanosensing. In the ROS project, we also take advantage of zebrafish embryos to image and manipulate early nervous system development in vivo. We use a combination of quantitative live cell imaging, cell biological, biophysical, molecular and biochemical techniques to investigate the dynamics and function of adhesion, signaling, and cytoskeletal proteins, and to quantify forces involved in cell migration. We hope that our research will not only improve our understanding of the basic cell biology of neuronal growth cones, but also assist in the development of treatments that support axonal regeneration in nervous system diseases and injuries. Specifically, we are conducting a drug screen with zebrafish larvae to identify compounds that promote regeneration following spinal cord injury. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdsuter@purdue.edu  |
| Swithers | Susan | Integrative Neuroscience |
Susan SwithersProfessor-Department of Psychological Sciences Current Research InterestsWork in my laboratory involves studying the development of controls ingestive behavior in rats. In particular, our work has recently focused on the acquisition of metabolic regulatory capacities, the role of ovarian steroids in modulating responses to changes in energy utilization and the role of relationships between sensory signals and calories in the modulation of food intake and energy balance. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactswithers@purdue.edu  |
| Szymanski | Daniel | Computational and Systems Biology, Plant Biology |
Daniel SzymanskiProfessor, Botany and Plant Pathology Current Research InterestsThe organization of the microtubule and actin filament cytoskeleton arrays plays an important role in defining cell shape and tissue development. However, there is very little mechanistic understanding of how the cytoskeletons are organized and regulated. For example, how do hormonal signals lead to cytoskeletal re-organization and a polarized growth response? What cellular intermediaries define cytoskeletal organization and pattern the cell wall during growth? The long-term goal of my research team is to analyze the multiscale control of growth from protein complexes to the morphogenetic control of cells, tissues, and organs. Arabidopsis leaf epidermal development is being used as an experimental model to better understand morphogenesis control. The leaf epidermis contains several highly polarized cell types that display unique responses to microtubule and microfilament disrupting agents. The lab is now using modern molecular genetic tools, multivariate live cell imaging, and finite element computational modeling of plant morphogenesis to discover how plants integrate cytoskeletal and cell wall systems during growth and development. The lab also has created new high throughput proteomic methods to analyze the location and composition of protein complexes in a variety of plant species, cell types, and differing genetic or environmental conditions.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdszyman@purdue.edu  |
| Talavage | Thomas | Integrative Neuroscience |
Thomas TalavageProfessor of Electrical & Computer Engineering Current Research InterestsPrimary research activities are related to elucidating the mechanisms underlying cumulative strain injuries associated with repetitive subconcussive blows in youth collision-sport athletes (see http://www.purdue.edu/research/png). Additional activities relate to better characterization of neurophysiologic health using multiple magnetic resonance-related neuroimaging modalities. I also continue to work in the auditory neurosciences, with an emphasis on enhancing the efficacy of electrical stimulation patterns, as generated by cochlear implants in response to external acoustic stimulation. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacttmt@purdue.edu  |
| Tao | Andy | Cancer Biology |
Andy TaoAssociate Professor Current Research InterestsLink to the group webpage: http://taolab.wikispot.org The mission of our research group is to bridge technology with biomedical/biochemical discovery. Mass spectrometry-based proteomics is the kind of research that is highly interdisciplinary, bringing together biology, chemistry, instrumentation, statistics, and bioinformatics. Proteomics thus holds significant promise for the discovery of diagnostic or prognostic protein markers, for the detection of new therapeutic targets and as a powerful tool to further our understanding of basic biological processes and mechanisms. The realization of these expectations relies on the development of novel chemistry and instrumentation. Our group focuses on the development of novel strategies and reagents to efficiently target and discover proteins of important biological relevance as potential biomarkers. Such proteins of interest are typically low in abundance, dynamically expressed, and post-translationally modified. The subject, called targeted proteomics, therefore involves the integration of a number of technologies including the selective targeting of proteins with activities of interest, multi-step sample preparation, and mass spectrometry. Examining changes in these proteins within cells under different physiological conditions will offer insights into understanding cellular and molecular mechanisms that cannot currently be obtained through traditional biological studies that usually focus on the detailed analysis of individual biomolecules. Current projects in my group are: 1. Quantitative and functional proteomics in vitro and in living cells using soluble nanopolymers; 2. Biomarker discovery using Ossabaw swine as the animal model for metabolic syndrome and cardiovascular disease; 3. Profiling of protease substrates in apoptotic cells; 4. Molecular signaling in cancer cells: phosphoproteomics. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacttaow@purdue.edu  |
| Teegarden | Dorothy | Cancer Biology |
Dorothy TeegardenProfessor Lead, Cancer Prevention and Control Program, Oncological Sciences Center Current Research InterestsMy research laboratory investigates mechanisms of disease progression and prevention of age-related diseases, particularly cancer. One area of focus is understanding the basic cellular mechanisms involved in regulating energy and lipid metabolism, including the roles that the enzyme pyruvate carboxylase (PC; in metastasis promotion) and vitamin D (in cancer prevention) play in the development of cancer and metastases. There is increasing recognition of the importance of the dysregulation of energy metabolism in cancer development. Although there is some evidence that the enzyme pyruvate carboxylase plays a role in cancer progression, particularly metastasis, little is known about the underlying mechanisms. Our studies are among the first to show that pyruvate carboxylase plays an essential role in fatty acid metabolism in metastatic cancer cells and is critical in the increased oxidative stress protection in cancer cells. We are also the first to show that pyruvate carboxylase is essential for breast cancer metastasis. These findings provide, for the first time, a mechanistic basis for the critical role of pyruvate carboxylase in cancer progression, particularly metastasis, and lay the foundation for understanding the metabolic shifts necessary to support metastasis. Substantial evidence suggests that vitamin D helps prevent the development of several cancers, including breast cancer. Our studies, which used appropriate cell models of cancer prevention, identified unique mechanisms by which 1,25 dihydroxyvitamin D inhibits processes essential to preventing cancer, including glucose and glutamine metabolism. Further, we showed that 1,25 dihydroxyvitamin D inhibits metastasis of breast epithelial cells, using a unique 3D cell culture model of breast to bone metastasis. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactdteegard@purdue.edu  |
| Tesmer | John | Cancer Biology, Chemical Biology, Biomolecular Structure and Biophysics |
John TesmerWalther Professor in Cancer Structural Biology Current Research Interests1) Molecular Basis of Heterotrimeric G Protein Function. Signal transduction conveyed from G protein-coupled receptors (GPCRs) via heterotrimeric G proteins is one of the classic paradigms of hormone action, wherein extracellular signals lead not only to transient changes in the concentrations of intracellular second messengers, but also to sustained changes such as chemotaxis, cell growth, and metastasis. Dr. Tesmer uses X-ray crystallography, cryo-electron microscopy and NMR to illuminate the mechanisms of heterotrimeric G protein signaling at atomic resolution.2) GPCR-Linked Rho Guanine Nucleotide Exchange Factors (RhoGEFs). Sustained changes in cell behavior induced by GPCRs typically involve modulating the actin cytoskeleton and gene transcription via RhoGEFs. These enzymes play a central role in chemotaxis, tumor growth and metastasis by activating Rho GTPases. In 2004, the lab reported atomic structures of the catalytic domains of leukemia-associated RhoGEF (LARG) alone and in complex with RhoA, and in 2006, the structures of the oncogenic Gα12 and Gα13 subunits that regulate the activity of LARG. In 2007, the lab resolved the structure of the Gαq-p63RhoGEF-RhoA complex, capturing a snapshot of a novel Gαq signaling pathway implicated in the development of cardiac hypertrophy. Most recently, the Tesmer lab has been characterizing the Rac1 specific enzyme known as P-Rex1, a Gβγ-regulated RhoGEF that plays a key role in neutrophil function and in metastasis of breast and prostate cancer, and Trio, a close relative of p63RhoGEF that is responsible for tumor growth in uveal melanoma. 3) Structure and Function of G protein-coupled receptor kinases (GRKs). The ~800 GPCRs in the human genome are regulated by a family of seven vertebrate kinases that inhibit signaling by activated GPCRs, ensuring a return to homeostasis. In 2003, the Tesmer lab published the structure of the GRK2-Gβγ complex, the first of a GRK and of Gβγ subunits in complex with an effector. In 2005, the Tesmer lab reported the structure of the Gαq-GRK2-Gβγ complex, providing a snapshot of activated Gα and Gβγ subunits at the membrane as they engage a common effector target. The structure also provided the first glimpse of Gαq. The lab went on to characterize GRKs from other subfamilies: GRK6, GRK1/rhodopsin kinase, and most recently GRK5. These structures helped to identify sites that form the docking site for activated GPCRs and for anionic phospholipids. Structural characterization of a GRK-GPCR complex remains an important goal. 4) Molecular Basis of Phospholipase Activation and Reverse Cholesterol Transport. The LPLA2/LCAT family of phospholipases transfers the sn2 acyl chain from lipids such as lecithin to acceptor lipids such as ceramide or cholesterol. These closely related enzymes are important for lung surfactant catabolism and reverse cholesterol transport to the liver via HDL, respectively. They are both important targets for biotherapeutic development. The Tesmer lab has determined crystal structures for both LPLA2 and LCAT and is working towards developing small molecular activators and variants with improved activity that could be used to treat acute coronary syndrome and/or fatal genetic diseases that inhibit LCAT activity, such as familial LCAT deficiency. 5) Identification and Rational Design of Small Molecule Probes. The Tesmer lab is interested in the discovery of selective small molecule agents that can probe the above signaling cascades in more physiological contexts or that can serve as leads for drug development. Most of our published work thus far focuses on GRK inhibitors, primarily targeting GRK2, which regulates cardiac output and hypertrophy and is overexpressed during heart failure. GRK2 inhibitors have potential applications ranging from treatment of congestive heart failure to inhibition of arterial and renal plaque formation. In 2012 the lab identified the selective serotonin reuptake inhibitor paroxetine (Paxil) as a moderately selective GRK2 inhibitor, which was later shown to reverse heart malfunction in mice subjected to infarction. Using a rational approach, we have identified even more potent chemical scaffolds with distinct selectivity profiles for the various GRKs in close collaboration with medicinal chemists. New small molecule screens targeting P-Rex1 and Trio are underway to identify leads for drugs that block GPCR-linked metastasis and tumor growth, respectively. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjtesmer@purdue.edu  |
| Thompson | David | Biomolecular Structure and Biophysics, Chemical Biology |
David ThompsonProfessor Current Research Interests
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdavethom@purdue.edu  |
| Tian | Shiliang | Biomolecular Structure and Biophysics, Chemical Biology, Integrative Neuroscience |
Shiliang TianAssistant Professor Current Research InterestsMetalloproteins are important in biology because they catalyze essential reactions in life-like cellular respiration, photosynthesis, and nitrogen fixation under mild conditions and with high efficiency and selectivity. Research in the Tian lab lies at the interface of physical, inorganic, and biological chemistry and utilizes a wide range of spectroscopic and computational techniques to elucidate the functions and mechanisms of metalloproteins. The information gained through this type of work will provide insight into the electronic and geometric structures of metalloproteins and their associated mechanistic roles in pathogenesis, which can be beneficial in the development of effective therapeutic treatments. We aim to 1) investigate the roles of metal ions homeostasis in neurodegenerative diseases, 2) design functional metalloproteins for biosynthetic, biotechnological and pharmaceutical applications, and 3) elucidate the molecular basis of the mechanism of magnetoreception.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsltian@purdue.edu  |
| Trader | Darci | Integrative Neuroscience, Microbiology, Immunology and Infectious Diseases, Integrative Neuroscience |
Darci TraderAssistant Professor Current Research InterestsThe Trader lab is focused on understanding the role of the proteasome in a variety of disease states. We are actively recruiting students for two projects. The first involves studying the role of the immunoproteasome, whose activity is essential for generating antigenic peptides. We wish to study how we can harness the activity of the immunoproteasome to elicit a desired immune system response. Students will utilize a variety of molecular biology techniques, flow cytometry, and confocal microscopy to study the immunoproteasome. The second project we are recruiting students for is to study how increasing the rate of proteasome activity can have a positive effect on a variety of protein accumulation diseases including Parkinson's, Alzheimer's, and premature aging. Students will use new molecules discovered in our lab in a variety of cell types to analyze the ability of the proteasome to degrade accumulated toxic proteins. Students will have the opportunity to learn confocal microscopy, advanced molecular biology techniques, and basic drug stability assays. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdtrader@purdue.edu 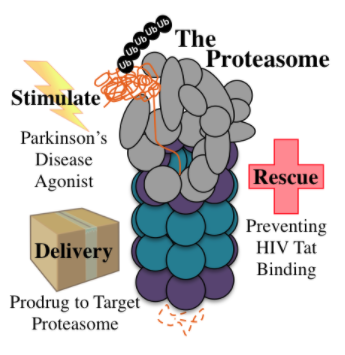 |
| Trask | Sydney | Integrative Neuroscience |
Sydney TraskAssistant Professor Current Research InterestsWe are interested in the ways the brain encodes, stores, retrieves, and updates memory. We are particularly interested in understanding memory for context, or the environment in which events take place. Successful encoding and retrieval of context allows us to select and guide our behavior in a way that encourages situationally appropriate responding. However, alterations in this type of learning and memory are common in symptomology that underlies several neuropsychiatric disorders, ranging from PTSD to age-related dementia. Understanding how memory for context is formed, retrieved, and altered at both the circuit and molecular levels will provide one crucial step forward to treatments aimed at reducing maladaptive behaviors stemming from contextually inappropriate responding.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactsmtrask@purdue.edu  |
| Umulis | David | Biotechnology, Cancer Biology |
David UmulisDane A. Miller Head and Professor of Biomedical Engineering Current Research InterestsThe focus of our research is to investigate the regulation of signal transduction in development. Specifically, we are interested in elucidating mechanisms of robustness, cell fate decisions, and tissue patterning by morphogen gradients. Engineers and biologists with some math/physics background are particularly capable of addressing these questions because development in many contexts relies on fluid flow, mass transport, chemical reactions, process control, and thermodynamics. One major goal of our lab is to foster interdisciplinary research projects to tackle problems in biology using quantitative image analysis and systems biology approaches such as mathematical models and bioinformatics. Much is known about the molecular components involved in signal transduction and gene expression in a number of model systems in developmental biology, and the focus is now shifting to understanding how these components are integrated into networks, and how these networks transduce the inputs they receive and produce the desired pattern of gene expression. The major question is how the correct genes are turned on at the correct point in space at the correct time in development to produce the numerous cell types present in an adult. While the problem is simply stated, delineating the mechanisms of development that impart the robust control requires sophisticated computational models. To study these problems, we focus on coupling advanced microscopy and image analysis with multi-dimensional finite-element models of biological patterning mechanisms: most recently BMP patterning of Drosophila embryos. The direct incorporation of experimental data into geometrically accurate computational models leads to significant new biological insights that cannot be gained by either approach independently. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactdumulis@purdue.edu  |
| Varala | Kranthi | Plant Biology |
Kranthi VaralaAssistant Professor Current Research InterestsThe Varala lab focuses on how plants perceive abiotic stress and alter their transcriptional programs as a response to the specific stress. We aim to capture natural genetic variation and the resultant transcriptional states leading to plant adaptation and resilience by using a combination of genomic, systems biology and phylogenetic tools. The research aims to understand how plants alter their transcriptional program immediately in response to thermal stress in the short term. We apply systems biology approaches in the model plants to understand transcriptional reprogramming in response to environmental stimuli such as temperature, nutrition etc. In the long term, we want to understand how plants change their overall gene expression regime on evolutionary scales to adapt to colder and warmer climates. We routinely use phylogenomic approaches to identify gene-to-trait association by tracking recurring changes in plant genomes that co-occur with the gain/loss of a trait. The broader question we aim to address, is whether the transient changes in the plant transcriptome offer insights into plant adaptation to diverse environments.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. ContactEmail: kvarala@purdue.edu
 |
| Verma | Mohit | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Mohit VermaAssistant Professor, Agricultural and Biological Engineering
Current Research InterestsMicrobiome: The complex communities of microorganims (i.e. microbiome)—bacteria, viruses, fungi, protozoa—that reside in and on us determine our health. Our lab is engineering these microbiomes to improve health and prevent diseases such as obesity, diabetes, cancer, and inflammatory bowel diseases. Nanotechnology: The use of nanotechnology permits us to manipulate biological matter (bacteria, viruses, fungi, mammalian cells) in novel ways. At the nanoscale, matter can have unique properties such as a change in color depending on its size (gold nanoparticles) or superparamagnetism (iron oxide nanoparticles). We harvest these properties to study and engineer microbiomes. User-friendly diagnostics: We are developing biosensors that are low-cost and user-friendly. These biosensors incorporate most of the complexity within the device (e.g. stored reagents) such that the user requires minimal training. Soft robots: The field of soft robotics has grown tremendously in the last decade. We are applying the soft actuators and robots developed so far to applications in maintaining human health and preventing injuries. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmsverma@purdue.edu
 |
| Volkening | Alexandria | Biomolecular Structure and Biophysics, Computational and Systems Biology |
Alexandria VolkeningAssistant Professor, Department of Mathematics (and Weldon School of Biomedical Engineering, by courtesy) Current Research InterestsMy team's broad research area is mathematical biology and applied math. We are interested in understanding how cells or other agents come together to create rich, group-level dynamics, particularly in developmental-biology settings. My primary focus has been investigating pigment-cell dynamics in zebrafish-skin patterns, with the goal of helping to identify links between genes, cell behavior, and phenotype. Our projects range from building experimentally-testable models for specific biological applications to developing mathematical methods for quantifying cell-based patterns, simulation frameworks, or data-driven methods for selecting models --- we draw on a wide range of mathematical approaches depending on where the driving biological question leads us. We're also always interested in building collaborations with experimentalists in new areas.
Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactavolkening@purdue.edu  |
| Waddell | Peter | Molecular Evolutionary Genetics |
Peter WaddellAssistant Professor-Biological Scienes Current Research InterestsI develop methods of analysis/algorithms, work on their implementation, apply them to data and gather data relevant to particular problems. My lab is both wet lab, focusing on RNA/DNA extraction, cell culture plus mass sequencing, and dry lab, focusing on computer analysis of biological data, especially genomic data. An important aspect of my research is to use experimental results combined with theory, to address key questions in evolutionary biology, bioinformatics and gene expression. The lab is currently actively recruiting at the graduate and postdoc level, in both computational and molecular biology. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactpwaddell@purdue.edu  |
| Watts | Val | Integrative Neuroscience, Membrane Biology |
Val WattsProfessor and Associate Dean for Research Current Research InterestsThe research in the Watts laboratory is designed to use a multi-disciplinary approach, combining molecular biology, biochemistry, and pharmacology to study the signaling mechanisms of G protein-coupled receptors (GPCRs) and the regulation of adenylyl cyclases. The fact that GPCRs are the target of more the 50% of today’s clinically used drugs emphasizes further the importance of these studies. Much of the work in his lab has focused on members of the dopamine, cannabinoid, serotonin, and adenosine receptor families. Studies have examined the pharmacology of these receptors including the characterization of novel ligands that activate these receptors as well as investigating their ability to modulate the activity of their primary effector, the enzyme adenylyl cyclase (AC). A second area of focus has included examining the effects of persistent Gαi/o-coupled receptor activation in vitro in order to understand and identify molecular changes following chronic drug exposure that may occur in vivo. Much of this work has focused on elucidating the mechanisms and pathways for D2 dopamine receptor-induced heterologous sensitization of adenylyl cyclase. Other exciting studies have used bimolecular fluorescence complementation (BiFC) to “visualize” and localize protein-protein interactions in living cells. BiFC is based on the complementation between fragments of fluorescent proteins that are fused to two interacting proteins. These fluorescent approaches and others are currently being used to study drug-modulated GPCR-AC, AC-AC, and AC-protein interactions in novel cellular models as well as in BiFC screening endeavors. More recent efforts have been focused on the development and execution of screening endeavors relevant to drug discovery and AC signaling. We have developed a number of new HTS assays for individual AC isoforms that include using siRNA and small molecule libraries. We are currently exploring the ability of selective inhibitors of AC isoforms to serve as non-opioid alternatives for chronic pain, agents to treat opioid withdrawal, and for the treatment of alcohol use disorders (AUDs).Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactwattsv@purdue.edu  |
| Weake | Vikki | Integrative Neuroscience |
Vikki WeakeAssociate Professor Current Research InterestsCurrent research in the lab of Dr. Weake focuses on understanding how aging contributes to ocular diseases such as age-related macular degeneration using Drosophila as a model system. The Weake lab use a combination of approaches including transcriptomics, epigenomics, mass spectrometry and imaging to study the mechanisms involved in neuronal aging. Her lab also has an interest in chromatin modifiers with a focus on characterizing novel subunits and types of Gcn5-containing histone acetyltransferase complexes. Please visit our lab website at purdue.ag/weakelab for more information. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactvweake@purdue.edu  |
| Wedow | Robbee | Computational and Systems Biology |
Robbee WedowAssistant Professor of Sociology and Data Science Current Research InterestsDr. Wedow's main research interest is in social science and behavioral genetics, which lies at the intersection of sociology, psychology, demography, and statistical & computational genetics. He is interested in large-scale genomics analyses, and also in how social forces and environments interact with genetics. He uses recent advances in genetic data collection, massive biobank-scale data, and methodological developments in statistical genetics to carry out his research. He is also deeply dedicated to clearly and sensitively communicating the findings from his work in an ethically-engaged and community-based fashion. He has written and taught widely on the ethical considerations and societal impacts of modern-day genomics research. He is actively involved in helping lead NSF funded work to restructure genetics curriculum in high school in order to be less deterministic, and instead to accurately reflect complex human variation. Dr. Wedow completed his PhD in Sociology and Statistical/Behavioral Genetics at the University of Colorado Boulder in 2018. He completed his postdoc in the Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard, in the Department of Sociology at Harvard University, in the Analytic and Translational Genetics Unit at Massachusetts General Hospital, and in the Department of Epidemiology at the Harvard T.H. Chan School of Public Health in 2022. His work outside of social science genetics focuses on population health, health disparities, and quasi-experimental designs and methodologies. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactrwedow@purdue.edu  |
| Wei | Alexander | Chemical Biology |
Alexander WeiProfessor and University Faculty Scholar Current Research InterestsResearch in the Wei group integrates organic chemistry, cell biology, and nanoscale materials science to develop new approaches to targeted delivery and therapy. Students in our group develop a wide range of research skills including organic and nanomaterials synthesis, optical and electron microscopy, and cell culture techniques. Some of our projects are described below. Nanomedicine: Gold nanorods, nanostars, and core-shell nanoparticles can be deployed as in vitro and in vivo probes for biosensing and biological imaging. These nanoprobes are functionalized with synthetic ligands for targeted delivery to tumor cells or microbial pathogens, and can be used simultaneously for diagnostic imaging and photoactivated therapies. The relatively new concept of "theragnostics" has now become an important paradigm in the emerging field of nanomedicine. Glycomimetic chemistry: Heparan sulfate and other cell-surface carbohydrate ligands contain unusual or 'left-handed' sugar residues, whose recognition may depend on a specific bioactive conformation or display of polar functional groups. We have developed synthetic methodologies providing access to new classes of compounds intended to mimic the recognition function of carbohydrates. These glycomimetics can be used to increase the specificity for cell adhesion and for the recognition of heparin-binding proteins, which have numerous roles in inflammation and cancer. We have also used this chemistry to prepare mirror-image carbohydrates, to explore the role of chirality in various aspects of glycobiology. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactalexwei@purdue.edu  |
| Weil | Clifford | Plant Biology |
Clifford WeilAssociate Professor-Department of Agronomy Current Research InterestsTransposons and DNA repair in maize, Arabidopsis and yeast The Genetics of Genetics: genes controlling meiotic recombination in plants Identifying Genetic networks by Mutant-Assisted Gene Identification and Characterization (MAGIC) Mutational redesign of maize starch: better health and biofuels The Maize TILLING Project: reverse genetics for crop functional genomicsTransposons and DNA repair in maize, Arabidopsis and yeast. We are interested in better understanding how plants organize, maintain and express their genomes. Our work examines how plant cells repair DNA breaks, a question that also addresses how genes introduced into plants can be targeted to their homologous chromosomal sites and how chromosomes recombine during meiosis. Transgenes generally incorporate at essentially random sites (often in the wrong chromosomal context) through a process of non-homologous DNA end-joining (NHEJ). A homologous recombination mechanism also works at very low levels in plants (though at higher levels in other organisms). We want to know how cells choose one mechanism over the other and how that choice might be manipulated so that maize and other crops can be modified in a more controlled manner. Our approach has been to study how NHEJ repairs the damage created when Ac/Ds transposons move in maize and Arabidopsis. We have also developed a system in which Ac/Ds transposons are mobile in yeast and other fungi; the damage they cause in yeast is also repaired by NHEJ, and we use yeast NHEJ genes and mutants to better understand the process in plants. Two key observations drive this research. 1) comparisons among datasets and with those from other plant species show that maize genome rearrangement and DNA repair have features unlike any other eukaryote. Deletion and mutation of bases is less severe in maize than in other species, suggesting maize has evolved more effective DNA repair responses to the presence of the active transposons maintained in its genome by human selection. We are using this difference in a comparative genomics study of NHEJ repair in plants, using Arabidopsis, maize, the maize progenitor teosinte, rice, tomato, barley and other species. We are also examining the effects of DNA repair mutations in our yeast transposition assay system. 2) When an Ac/Ds element excises, the host DNA flanking the transposon forms a DNA hairpin structure, with the backbone of one strand of the double helix covalently bound to the backbone of the complementary strand. This structure is the same one formed when vertebrate immunoglobulin gene segments rearrange to make antibody-encoding genes. Plants do not have homologs of some of the genes essential to this process in vertebrates, yet they repair DNA hairpins very effectively. We want to know what these differences can teach us about how to treat particular forms of immune disease in animals. The Genetics of Genetics: genes controlling meiotic recombinationMeiotic recombination is another important aspect of DNA breaking and rejoining. In maize, most recombination events (“crossovers”) occur within or very near genes rather than in the repetitive retroelements that comprise 80% of the maize genome. In addition, meiotic crossovers are generally limited to one per chromosome arm through a poorly understood process called crossover interference. An interesting correlation is also that transposons such as Ac/Ds insert preferentially into the same regions where recombination events take place. As part of two collaborative efforts involving six other institutions, we have employed a forward genetic screen to identify over 100 mutants in maize that increase the frequency of recombination events, that decrease recombination, and that reduce crossover interference, a well as a reverse genetic screen to look at mutations in maize and Arabidopsis homologs of known recombination genes. We want to determine whether mutants identified in these screens are global or affect only specific regions of the genome, what effects these mutations have on the number and distribution of the protein machinery that carries out crossover events, (“recombination nodules”), and whether the distribution and frequency of transposon insertion is altered. Identifying Genetic Networks by Mutant-Assisted Gene Identification and Characterization (MAGIC)Geneticists have long noticed that a mutation crossed into different genetic backgrounds show a range of expression levels. These so-called “background effects” are often a complication in characterizing new mutations. However, the differences in expression also reflect interactions between the mutation in question and other genes in the genome, the very genetic networks that a lot of current work is trying to reveal. Collaborating with Guri Johal at Purdue and Peter Balint-Kurti of the USDA-ARS, we are characterizing these genetic networks by taking advantage of the fact that diverse maize inbred lines differ at, on average, ~1.5% of their sequence. The Panzea Project has identified 25 maize inbred lines that, among them, represent >80% of the genetic diversity known in maize. Furthermore, sets of 200 recombinant inbred lines (RILs) have been made for each of these lines crossed to the sequenced, reference B73 inbred line. The 5000 resulting lines, known as the Nested Association Mapping (NAM) panel, have been and continue to be genotyped extensively; these data are publicly available. The RILs partition the genomes of each diverse parent into well-characterized segments. Crossing known mutations in the B73 inbred to the 25 NAM founder lines, we examine progeny for enhanced or suppressed expression of the mutant phenotype. For any founder lines where we observe genetic modifiers of our starting mutation, we go back and cross the mutation to the 200 RILs derived from that parent, again screening progeny for the same enhanced or suppressed phenotype we observed with the diverse parent line of these RILs. The segments of the diverse parent that those RILs have in common then identify candidate regions for the modifiers. We call this approach Mutant-Assisted Gene Identification and Characterization (MAGIC) and have been able to demonstrate its utility using the dominant lesion mimic mutation Rp1D21, as well as recessive alleles of tie-dyed1 (tdy1), tdy2 and sucrose export defective1 (sxd1), which are unable to move sucrose out of the leaves effectively after photosynthesis (a collaboration with David Braun at Univ. of Missouri). Additional studies are looking at modifiers of kernel starch and protein as well as kernel development mutants. Mutational redesign of maize starch: better health and biofuelsWe are using a combined genetic, biochemical and protein structural approach to look at how specific mutations in maize alter starch digestibility. Cornstarch that digests more slowly in its cooled form and therefore releases glucose into the bloodstream over a longer period than normal cornstarch can be valuable in combating obesity and diseases related to it, as well as Type II diabetes. Conversely, starch that digests rapidly without the need for cooking can improve the efficiency of producing biofuels such as ethanol and butanol. We have identified ~100 mutant lines that alter starch digestibility, including those that decrease digestibility and those that increase digestibility. We are initiating more detailed characterization of these mutations and, in collaboration with the Whistler Center for Carbohydrate Research (located at Purdue), characterizing what they do to starch fine structure, to the interaction of starch with other cellular components and to the ultrastructure and development of the starch granule. Additional biofuels projects include modifying maize kernel architecture and developing maize lines that accumulate sugars in the stalk. The Maize TILLING Project, one of the most powerful tools available for understanding gene function is to analyze the effects of mutating that gene. We operate the Maize TILLING Project, an NSF-supported resource, as a service to the international maize community. TILLING is a technology, originally developed for Arabidopsis, that allows us to screen through the DNAs of a large, EMS mutagenized population of maize and quickly identify any individuals in that population that have a mutation in a user’s gene of interest. We then sequence the mutations we find and return this information, the predicted effects of the mutations and, most importantly, seed carrying the mutations to the person who initiated the request. These mutants can then be analyzed further to better understand gene function. We are developing projects that apply this technique to other crops as well, including soybean, sorghum, marigold, switchgrass and ryegrass, and welcome inquiries into its application to other crops. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactcweil@purdue.edu  |
| Wendt | Mike | Cancer Biology |
Mike WendtAssociate Professor Current Research InterestsResearch in the Wendt lab focuses on pharmacological targeting of molecular players involved in the metastatic outgrowth of breast cancer. Systemic dissemination is an extremely early event in breast cancer progression, therefore we primarily focus on developing strategies that are specifically designed to target secondary tumors. We take two major approaches to this challenge: 1.Through metastatic progression breast cancer cells under epithelial-mesenchymal transition (EMT) and reverse process of mesenchymal-epithelial transition (MET). An overriding hypothesis in the lab is that through the processes of EMT:MET the hybrid epithelial states that characterize the metastatic tumor are fundamentally unique from the epithelial status of the primary tumors. We believe these differences hold the keys to developing therapeutics capable to pharmacologically treating metastatic lesions. 2.Subsequent to dissemination, a large fraction of tumor cells enter into an asymptomatic state of dormancy. The ability of normal organs to resist secondary tumor formation is the body’s last defense against metastatic disease progression. The environmental factors (drugs, alcohol, diet, etc…) capable of “awaking” disseminated cells are poorly characterized. Understanding these factors is required if we hope to design therapeutics capable of maintaining systemic tumor dormancy. We use a combination of molecular, biochemical, cell biological and whole animal studies to evaluate the impact of anticancer therapies on EMT and metastatic progression. Specifically, we utilize a variety of 3D and multicellular colculture models to evaluate growth factor signaling, pharmacological response to anti-cancer drugs, and as a platform for genetic and compound screening assays. In addition to these in vitro approaches we have developed novel in vivo mouse models of metastasis and drug resistance. We utilize these models in combination with bioluminesent imaging as a robust approach to locate and quantify metastatic progression. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactmwendt@purdue.edu  |
| Wilker | Jonathan | Chemical Biology |
Jonathan WilkerAssociate Professor of Chemistry and Materials Engineering Current Research InterestsVisit our research group web site here: http://www.chem.purdue.edu/wilker/ Biological Materials from the Oceans We are exploring how biological systems produce materials. Our focus is upon marine biomaterials such as mussel adhesives, barnacle cements, and oyster reef structures. Efforts include characterizing marine biological materials, designing synthetic mimics, and developing biomedical applications such as surgical adhesives. In the case of mussel adhesive we know that the material is generated by cross-linking a series of proteins into the final, cured matrix. We are using live animals, extracted protein, and peptide models to characterize formation of this material and determine the bonding contained within. For other organisms such as barnacles and oysters we are working to extract and characterize the protein components of these materials. Microscopy also helps to shed light on how the animals adhere. With characterization insights in hand we can then develop synthetic polymer mimics of these biological materials. Such new classes of polymers cross-link in a manner analogous to the proteins. We have achieved strong adhesion using these polymers, with bonding abilities comparable to commercial “super” glues. Bioinspired polymers make good candidates for some of the most in-demand biomedical materials. At the moment there are no surgical adhesives available that are simultaneously wet setting, strong bonding, and non-toxic. Marine biology may have already solved this problem, hence the exploration of these materials for biomedical applications. We are using our new bioinspired materials to bond together both soft and hard substrates such as skin and bone as part of efforts to develop the next class of biomedical adhesives. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactwilker@purdue.edu  |
| Wisecaver | Jennifer | Plant Biology, Computational and Systems Biology |
Jennifer WisecaverAssistant Professor, Department of Biochemistry Current Research InterestsResearch in the Wisecaver lab is focused on the genomic basis of evolutionary innovation, which we study in plants, fungi, and eukaryotic microbes. By integrating our findings across these diverse lineages, we aim to evaluate the timing, consequence, and generality of different genetic mechanisms underlying the evolution of novel traits in organisms. The traits that we focus on—specialized metabolites—allow organisms to interact with and manage the world around them. For example, land plants, being sessile and at the mercy of their surroundings, produce specialized metabolites to resist abiotic stress, attract pollinators and seed dispersers, and combat pathogens and herbivores. Similarly, fungi, which digest their food externally and must guard these nutrients from competitors, produce many potent antimicrobial metabolites. Specialized metabolic pathways are often the business end of evolutionary arms races; as one species evolves a toxic pathway, a competing species evolves complementary resistance. Biochemical surveys of species from across the tree of life suggest that the number of specialized metabolic pathways is enormous. Yet, because of their fast-evolving nature, nearly all of these pathways are unresolved at the genetic level, which has slowed our ability to draw general conclusions about their evolution. Thanks to amazing advances in sequencing technology and computational biology, work in the lab aims to accelerate the pace of research in this area. Currently, the lab uses 1) functional genomics & network biology to identify the genes responsible for making specialized metabolites followed by 2) comparative genomics & phylogenetics to untangle the genes' evolutionary history. Questions we ask include: What is the relative contribution of gene duplication, horizontal gene transfer, and other sources of innovation such as de novo gene formation in the birth of new metabolic pathways? What is the speed and importance of gene innovation at the regulatory versus enzymatic level? How does the impact of these processes vary across environments and between lineages? Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactjwisecav@purdue.edu  |
| Yang | Danzhou | Cancer Biology, Biomolecular Structure and Biophysics |
Danzhou YangMartha and Fred Borch Chair in Cancer Therapeutics, Professor Medicinal Chemistry and Molecular Pharmacology Current Research InterestsResearch in my laboratory is focused on structures and functions of cancer-specific DNA molecular targets and structure-based rational drug design. Particularly, we work on G-quadruplex (G4) DNA secondary structures formed in human oncogene promoters and human telomeres, which are attractive new molecular targets for cancer therapeutics. We seek to understand structure-function relationships of DNA G-quadruplexes, their protein interactions and drug targeting. In addition, we work on DNA-targeted anticancer drugs that inhibit transcription factors and topoisomerases. We use a combination of structural, biophysical, biochemical, molecular, and cellular methods in our research, in particular high-field NMR spectroscopy for biomolecular structural study. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyangdz@purdue.edu  |
| Yang | Yang | Computational and Systems Biology, Integrative Neuroscience |
Yang YangAssistant Professor, Medicinal Chemistry and Molecular Pharmacology Current Research InterestsAutism, epilepsy, social behaviors, induced pluripotent stem cells, transgenic mouse models, optogenetics, chemogenetics, and brain tumors. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyangyang@purdue.edu  |
| Yeo | Yoon | Biotechnology, Microbiology, Immunology and Infectious Diseases |
Yoon YeoProfessor, Industrial and Physical Pharmacy Current Research InterestsNanoparticle surface engineering for target-specific drug delivery Anion-resistant non-viral gene vectors Modulation of tumor microenvironment for cancer immunotherapy Drug delivery systems for treatment of sepsis and infectious diseases Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyyeo@purdue.edu  |
| Yoon | Gyeong Mee | Cancer Biology, Plant Biology |
Gyeong Mee YoonAssistant professor Current Research InterestsDr. Yoon’s research interest lies in understanding of the molecular mechanisms of the key steps in the phytohormone ethylene biosynthesis and its signaling pathway using the model plant Arabidopsis thaliana. The gaseous hormone ethylene regulates many important plant growth and developmental processes, including seed germination, root hair formation, nodulation, senescence and response to a variety of developmental and environmental stresses. Two main research projects are currently under way in the lab. First is to investigate the molecular mechanism regulating protein turnover in ethylene biosynthesis. Specifically, we are aiming to understand the roles of phosphorylation in protein turnover of the 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), a key enzyme in the ethylene biosynthesis and the Ethylene Overproducer 1 (ETO1)/ETO1-like (EOL) proteins, ubiquitin ligases that target a subset of ACS for degradation. Besides, we are also interested in identification and characterization of novel proteins regulating ethylene biosynthesis to acquire better insight in the molecular aspects of ethylene biosynthesis regulation. Secondly, we are focusing on elucidation of the roles of Constitutive Triple Response 1 (CTR1), a Raf-like mitogen activated protein kinase kinase kinase (MAPKKK) that acts as a negative regulator, in the ethylene signaling pathway. Ethylene is perceived by a family of ethylene receptors that are derived from two-component histidine kinase, and the receptors are located at the Endoplasmic Reticulum (ER). Recently, we resolved one of the longstanding questions in the ethylene signaling fields how ethylene signals are transduced from the ER to the nucleus to activate ethylene responsive genes in the nucleus. In the absence of ethylene, CTR1 phosphorylates EIN2, a critical positive regulator in the pathway, which blocks an activating proteolytic cleavage. In response to ethylene, the inactive CTR1 fails to phosphorylate EIN2, which is then cleaved by an unidentified protease, releasing the C-terminal domain that then migrates into the nucleus where it activates the transcription factor EIN3, either directly or indirectly, to regulate ethylene-mediated gene expression. Elucidation of the mechanism for regulating EIN2 in the ethylene signaling pathway bridges the gap between the signaling events from the receptor at the ER to the nucleus. However, this also raises a number of intriguing questions and our lab is particularly interested in understanding: 1) how do the ethylene receptors activate CTR1 in the early step of the ethylene signaling pathway?; 2) how does the ethylene response induced by the activation of EIN2 is turned off in the nucleus?; 3) and what are the roles of CTR1 other than phosphorylating EIN2 at the ER. We use the combination of biochemistry, genetics, cell biology and molecular biology approaches to address these questions. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactyoong@purdue.edu  |
| Zelaznik | Howard | Integrative Neuroscience |
Howard ZelaznikProfessor of Health and Kinesiology Current Research InterestsProfessor Zelaznik studies the coordination of bimanualperformance, such as drawing lines and circles; theories and models for speed-accuracy trade-off behavior; individual differences in timing and spatial ability; development of stable modes of coordination; motor performance of individuals who stutter as well as motor control in American Sign Language. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contacthnzelaz@purdue.edu  |
| Zhang | Cankui | Biotechnology, Plant Biology |
Cankui ZhangAssociate Professor Current Research InterestsLong distance signaling; plant mineral molecular biology Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactckzhang@purdue.edu  |
| Zhang | Guangjun | Cancer Biology, Computational and Systems Biology Integrative Neuroscience |
Guangjun ZhangHayward Associate Professor, Department of Comparative Pathobiology Current Research InterestsOur current research is focusing two major directions: 1). Developmental patterning roles of bioelectricity and 2). Novel cancer driver gene discovery using comparative genetic approach. In addition, we are also interested in the developmental mechanisms of vertebrate morphological novelties during evolution (Evo-Devo), especially the roles of gene/genome duplications in early vertebrates. 1). Developmental patterning roles of bioelectricity and ion channels. Bioelectricity refers to endogenous electrical signaling mediated by ion channels and pumps located throughout the cell membrane. Cell membranes act as electrical insulators, in part, due to the phospholipid bilayer’s impermeability to ions. Any ion that forms a gradient across a membrane will contribute to the actual membrane potential and bioelectrical signaling. It is already known that mutations or malfunctions of potassium channel genes cause a variety of diseases in the central nervous system, heart, pancreas, and kidneys (long-QT syndromes, episodic ataxia, familial convulsions, etc.). However, the function of ion channels and bioelectricity are just being recognized in developmental biology. There is growing evidence that bioelectricity also plays an important role in vertebrate embryogenesis, wound healing, and cancer. Currently, we are focusing on the developmental roles of potassium channel genes zebrafish fin size and pigment patterns. 2). Novel cancer driver gene discovery using comparative oncogenomics. Most human cancer cell genomes contain thousands of genetic alterations. But not all the altered genes equally contribute to cancer development and progression. A major goal of current cancer research is to distinguish pathogenetically relevant genetic alterations (drivers) from the passive changes (passengers) in cancer genome, thus targeting therapy can be developed on human tumors. Our lab mainly uses zebrafish as a model to study human cancer biology of MPNSTs (malignant peripheral nerve sheath tumors). First, novel human cancer driver genes will be identified through zebrafish-human comparative oncogenomic analysis and zebrafish genetic models. Following identification, novel genes’ functions and mechanisms will be examined in cancer and vertebrate development. development. The new cancer driver genes will provide more information for not only cancer biology, but also guides on human cancer patient diagnosis, treatment and prognosis. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactgjzhang@purdue.edu  |
| Zhang | Lei | Plant Biology, Chromatin and Regulation of Gene Expression, Plant Biology |
Lei ZhangAssistant Professor Current Research InterestsMolecular plant-nematode interactions.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactleizhang@purdue.edu  |
| Zhang | Min | Biotechnology, Computational and Systems Biology |
Min ZhangAssistant Professor of Statistics Current Research InterestsMy research interest is to develop new statistical approaches for high dimensional data involved in biological research. Currently I am focusing on developing methods for genome-wide association study and quantitative trait loci mapping. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactminzhang@purdue.edu  |
| Zhang | Zhong-Yin | Cancer Biology, Chemical Biology. Biomolecular Structure and Biophysics |
Zhong-Yin ZhangDistinguished Professor and Head, Medicinal Chemistry and Molecular Pharmacology, Director, Purdue Institute for Drug Discovery Current Research InterestsStructure/Function, Signaling Mechanism, and Therapeutic Targeting of Protein Tyrosine Phosphatases Aberrant cellular signaling stemming from altered protein tyrosine phosphorylation is a major contributing factor to human diseases. Consequently, anomalous cellular events driven by defective tyrosine phosphorylation afford tremendous opportunities for targeted intervention. This is evident by the abundance of kinase-based therapeutics that have become important cancer treatment modalities. Given the reversible nature of protein tyrosine phosphorylation, it is conceivable that disease progression could be halted by modulating the activity of protein tyrosine phosphatases (PTPs). However, despite increasing attention to the PTPs, they still remain largely an underexploited target class. Among factors that contribute to the difficulty of PTP-based drug discovery are lack of detailed understanding of how PTP malfunction causes diseases and insufficient target validation. In addition, the PTPs are exceptionally challenging targets for the development of potent and selective small molecule inhibitors due to the highly conserved and positively charged active sites. Research in this laboratory spans the disciplines of chemistry and biology with an emphasis on the structure and function of protein tyrosine phosphatases (PTPs), roles of PTP in normal physiology and pathological conditions, and the design and synthesis of PTP inhibitors as chemical probes to interrogate PTP function and as novel therapeutics for the treatment of cancer, diabetes and obesity, autoimmune disorders, neurodegenerative and infectious diseases. To understand the function of PTPs, we utilize biochemical, cellular, genetic, and proteomic approaches to probe the roles of PTPs in cellular signaling. Specifically, we carry out detailed mechanistic and kinetic study of PTP catalysis and substrate recognition using physiological substrates. Understanding the molecular basis for tyrosine dephosphorylation by PTPs will open doors to new experimental approaches that will elucidate mechanisms by which these enzymes control cell functions. We employ high-affinity PTP substrate-trapping mutants in combination with mass spectrometry for rapid isolation, identification, and characterization of physiological PTP substrates. Identification and characterization of cellular PTP substrates will help elucidate the function of individual PTPs as well as assignment of PTPs to specific signaling pathways. We design activity-based probes to analyze globally PTP activity both in normal physiology and in pathological conditions. The ability to profile the entire PTP family on the basis of changes in their activity should greatly accelerate both the assignment of PTP function and the identification of potential therapeutic targets. We also employ state-of-the-art molecular and mouse genetic techniques (e.g. CRISPR gene editing, siRNA silencing, and gene knockout) to define the roles of PTPs in normal physiology and in diseases. To facilitate therapeutic targeting of the PTPs, we have established a unique academic chemical genomic program encompassing high-throughput screening, structure-based design, and medicinal chemistry to develop small molecule PTP probes for functional interrogation, target identification/validation, and therapeutic development. To this end, we have pioneered a novel paradigm for the acquisition of potent and selective PTP inhibitors by targeting both the PTP active site and unique pockets in the vicinity of the active site. We have developed a number of nonhydrolyzable pTyr pharmacophores that are sufficiently polar to bind the PTP active site, yet remain capable of efficiently crossing cell membranes, offering PTP inhibitors with both high potency and excellent in vivo efficacy in animal models of oncology, diabetes/obesity, autoimmunity, and tuberculosis. Current efforts aim to advance our lead generation paradigms and to create a ‘PTP-based drug discovery platform’ that will ultimately impact broadly the portfolio of tomorrow. Students will have the opportunity to interact with a highly interactive, collaborative and multi-disciplinary group of individuals with expertise ranging from biochemistry and cell biology, mouse genetics, structural biology, chemical biology and medicinal chemistry. Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactzhang-zy@purdue.edu  |
| Zhang | Wei | Integrative Neuroscience |
Wei ZhangProfessor of Health Sciences and Toxicology Current Research Interests(1). To explore mechanisms by which lead (Pb) exposure alters beta-amyloid transport by brain barrier systems and affects the homeostasis of beta-amyloid in the central milieu, leading to cerebral amyloid angiopathy (CAA) in Alzheimer’s disease. (2). To explore the role of copper (Cu) in adult neurogenesis in subventricular zone (SVZ) by exploring the mechanisms by which the choroid plexus regulates Cu homeostasis in brain as it influences the neuronal activity in the SVZ. (3). To explore mechanisms, biomarkers and chelation therapy of manganese (Mn)-induced Parkinsonian disorder, by using laboratory animal models, human cohorts and noninvasive technical assessment of cumulative Mn in human bones. PULSe Contributor - not currently hosting students for laboratory rotations or recruiting students in the laboratory. Contactwzheng@purdue.edu  |
| Zhou | Daoguo | Microbiology, Immunology and Infectious Diseases |
Daoguo ZhouProfessor of Biological Sciences Current Research InterestsPathogenic Salmonella strains cause food poisoning, gastrointestinal inflammation, typhoid fever, and septicemia in humans. The bacteria achieve this by making a panel of specialized proteins that allows it to invade non-phagocytic intestinal epithelial cells, the first step in the disease process. Previous research has established that upon contact with the intestinal epithelial cells, Salmonella injects a set of proteins into the host cells through the bacterially encoded type III protein secretion system. These proteins subvert host cell signal transduction pathways to induce profuse actin cytoskeletal rearrangements and membrane ruffling, leading to the uptake of the bacterium. This uptake process is a highly regulated event and requires the coordinated action of the injected bacterial proteins as well as host proteins. My laboratory is interested in identifying and characterizing bacterial and host cellular proteins that are involved in Salmonella -induced actin cytoskeletal rearrangements, especially cellular proteins whose activities are modulated by Salmonella proteins. Studying the interaction between bacteria and their hosts has emerged as one of the most exciting areas of cellular microbiology. It will not only broaden our understanding of bacterial pathogenesis, but also will provide unexpected insights into basic host cellular functions, such as the cytoskeletal rearrangements and signal transduction pathways controlling cell movement.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contactzhoud@purdue.edu  |
| Zhou | Yun | Plant Biology |
Yun ZhouAssistant Professor, Department of Botany and Plant Pathology, Center for Plant Biology
Current Research InterestsThe research interest of Dr. Zhou’s group is to understand cellular and molecular mechanisms in control of plant meristem development, using both experimental and computational approaches. Our research centers on the stem-cell identity and function during plant development, and we are interested in understanding: how stem cells are dynamically organized and maintained through continuous cell division, growth and differentiation; how developmental decisions are made during cell patterning and new organ formation; and how the transcriptional circuits mediate stem-cell homeostasis and cell-cell communication. We are tackling these questions using multiple interdisciplinary methods, including live-cell imaging, computational quantification, modeling, biochemistry, molecular genetics, and functional genomics.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact765-494-2069
LILY B472
 |
| Zhou | Yun | Plant Biology |
Yun ZhouAssistant Professor, Department of Botany and Plant Pathology, Center for Plant Biology
Current Research InterestsThe research interest of Dr. Zhou’s group is to understand cellular and molecular mechanisms in control of plant meristem development, using both experimental and computational approaches. Our research centers on the stem-cell identity and function during plant development, and we are interested in understanding: how stem cells are dynamically organized and maintained through continuous cell division, growth and differentiation; how developmental decisions are made during cell patterning and new organ formation; and how the transcriptional circuits mediate stem-cell homeostasis and cell-cell communication. We are tackling these questions using multiple interdisciplinary methods, including live-cell imaging, computational quantification, modeling, biochemistry, molecular genetics, and functional genomics.Active Mentor - currently hosting PULSe students for laboratory rotations and recruiting PULSe students into the laboratory; serves on preliminary exam committees. Contact765-494-2069
LILY B472
 |

