
| RELATED WEB SITES |
| * Purdue structural biology |
| * Purdue Department of Biological Sciences |
| RELATED NEWS RELEASE |
| * Purdue biologists clarify how a cellular 'spacecraft' opens its airlock |

November 30, 2007
Purdue researchers obtain a snapshot clarifying how materials enter cells
WEST LAFAYETTE, Ind. -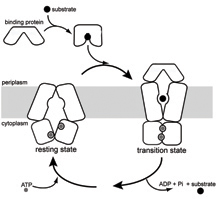 |
A research team led by Jue Chen, an associate professor of biological sciences, obtained a snapshot of the tiny protein gate complex that opens and closes pathways through the protective cellular membrane. The gates, operated by small protein machines that push them open and closed, bring nutrients into the cell and flush out waste.
The Purdue-led team was the first to achieve an image of the middle step of the process, capturing the molecular interactions as material passes through the membrane.
"By understanding the mechanisms of this process, researchers may be able to design more effective treatments for diseases that involve this group of proteins, such as cancer and cystic fibrosis," said Chen, who also is a member of Purdue's structural biology group within the College of Science. "With this knowledge, researchers may be able to inhibit or activate this mechanism, depending on what is needed to counteract the disease. For instance, many cancer cells are resistant to drug treatments because the cells pump the drugs out through these channels before they can work."
Amy Davidson, who collaborated on this work with Chen, said capturing an image of the intermediate stage is a giant step toward learning the complete process.
 |
"If you look at only the before and after stages, you don't really know all that goes on," said Davidson, an associate professor of chemistry at Purdue. "The intermediate stage provides all of this information about how the process really works. It shows all of the main components of the system interacting, which had not been seen before. It is a snapshot of what happens halfway through the entry process and is a very clear picture of how things work."
The research team used X-ray crystallography to obtain a picture of a special protein, called an ABC transporter protein, as it moved material through the cellular membrane. The work was published in last week's issue of Nature.
In addition to Davidson, Chen's research team includes postdoctoral research associates Michael Oldham and Dheeraj Khare from Purdue, and professor Florante Quiocho from Baylor College of Medicine. The team began this work in 2001.
ABC proteins are present in every living thing and have important biological functions. Scientists have identified 49 different ABC proteins in humans and have found that more than a dozen disease states are associated with malfunctions of these proteins, including macular dystrophy and problems in the regulation of cholesterol and insulin secretion.
Chen's team isolated ABC proteins from an E. coli bacterium, which is the standard research subject for this field of work. The ABC proteins are structurally very similar to those in human cells, and most of the principles can be directly applied to humans, Chen said.
Membrane proteins are notoriously difficult to study, Chen said. While most proteins dissolve in water and can be easily crystallized and examined, membrane proteins dissolve only in fatty substances, making it hard to isolate them for study.
The collaboration between Chen and Davidson, a structural biologist and a biochemist, was the key to success in capturing the intermediate structure, Chen said.
Davidson identified a special mutant of the ABC protein that locks halfway through the process. The mutant trapped the protein complex in a stable form that allowed Chen to crystallize and visualize the structure.
"We have the common goal to learn how this family of proteins works, but we take different approaches," Chen said. "It is important to communicate with scientists in other fields who can offer clues and tools to help reach your goal. We used genetic data and biochemical data to learn what we needed to crystallize and solve the structure."
While structural biologists attempt to obtain images of a protein as it performs a process, biochemists use indirect methods to understand the nature of a protein and how it moves through different conformations.
"Collaboration allows us to marry visual structures to other techniques that let you see the motions," Davidson said. "It all came together beautifully and matched the model we proposed in 2001 of what things should look like during this process. Through genetic and biochemical work we determined which proteins were important to this process, but we didn't know exactly how they worked. The image of the structure answers these questions and clearly shows the specific interactions."
Next, the team will work to determine the structure of another conformation of the protein that explains the other half of the process: how material is delivered into the cell.
Chen's research group is associated with the Purdue Cancer Center, one of just seven National Cancer Institute-designated basic research facilities in the United States. It was established in 1976 and attempts to help cancer patients by identifying new molecular targets and designing future agents and drugs for effectively detecting and treating cancer. The center also is affiliated with the Oncological Science Center in Purdue's Discovery Park.
Chen and Davidson also are members of Purdue's Center for Basic and Applied Membrane Sciences. The center was established to utilize the expertise of 45 faculty members. The center fosters collaboration in 12 different areas of basic and applied membrane sciences, with the mission to improve basic biochemical and biophysical understanding of membrane sciences, and to develop applications that are important in medicine, physiology and engineering.
The National Institutes of Health, Welch Foundation, Pew Scholar Program and a postdoctoral fellowship from the American Heart Association supported this work.
Writer: Elizabeth K. Gardner, (765) 494-2081, ekgardner@purdue.edu
Sources: Jue Chen, (765) 496-3113, chenjue@purdue.edu
Amy Davidson, (765) 494-5291, adavidso@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
GRAPHIC CAPTION:
This graphic illustrates an intermediate step in the process that brings nutrients or other substances into cells. The membrane proteins are shown holding the nutrient in a small cavity within the cellular membrane. A Purdue team led by Jue Chen, an associate professor of biological sciences, captured an image of a key step that reveals interactions of the main proteins involved in the process. (Purdue graphic/Chen labs)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2007/chen-illust.jpg
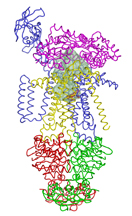
IMAGE CAPTION:
This image shows the conformation of proteins around a nutrient as it is transported across the cellular membrane. Each color represents a different protein, and the shaded area in the center shows a cavity containing the nutrient. Purdue associate professor of biological sciences Jue Chen led the team that was the first to obtain an image of this structure. (Purdue graphic/Chen labs)
A publication-quality photo is available at https://www.purdue.edu/uns/images/+2007/chen-illust2.jpg
Crystal Structure of a Catalytic Intermediate of the Maltose Transporter
Michael L. Oldham, Dheeraj Khare, Florante A. Quiocho, Amy L. Davidson & Jue Chen
The maltose uptake system of E. coli is a well-characterized member of the ATP-binding cassette transporter superfamily. Here we present the 2.8A crystal structure of the intact maltose transporter in complex with the maltose-binding protein, maltose and ATP. This structure, stabilized by a mutation that prevents ATP hydrolysis, captures the ATP-binding cassette dimmer in a closed ATP-bound conformation. Maltose is occluded within a solvent-filled cavity at the interface of the two transmembrane subunits, about halfway into the lipid bilayer. The binding protein docks onto the entrance of the cavity in an open conformation and serves as a cap to ensure unidirectional translocation of the sugar molecule. These results provide direct evidence for a concerted mechanism of transport in which solute is transferred from the binding protein to the transmembrane subunits when the cassette dimmer closes to hydrolyse ATP.
To the News Service home page
If you have trouble accessing this page because of a disability, please contact Purdue News Service at purduenews@purdue.edu.
