 Purdue Science & Health Briefs
Purdue Science & Health Briefs
 Purdue Science & Health Briefs
Purdue Science & Health Briefs
|
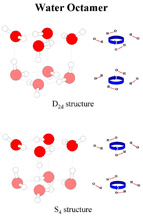
In addition, these tiniest cubes came in two forms, which had the same mass and structure, but differed in the arrangement of the hydrogen bonds within the cubes.
The study, which appears in the June 13 issue of the journal Science, was conducted by chemist Timothy Zwier, postdoctoral associate Caleb Arrington, and graduate students Christopher Gruenloh and Joel Carney.
"These findings verify what theorists have predicted for years; namely, that the eight water molecules preferentially form a cubic structure," Zwier says. "It also provides the first evidence that even in very small water clusters, water has the capacity to arrange its hydrogen bonds in several distinct orientations, much as it does in forming the many different solid phases of ice."
The study gives the first glimpse of nature's tiniest ice cubes and provides new information on the unique ability of water to hydrogen bond to itself to form large networks. This ability gives water many of its unique properties, including the unusual capacity of solid ice to float on liquid water.
Water, known as the "universal solvent," also has the unusual ability to dissolve a variety of substances. "Our understanding of water is so important because all biological processes, including those occurring in the human body, take place in a water-based solution", Zwier says.
What makes water unique are the hydrogen bonds that form between water molecules and other molecules. Each V-shaped molecule of water contains one oxygen atom centered between two hydrogen atoms. The molecule is held together by chemical bonds that create a slightly negative charge on the oxygen atom and a small positive charge on each of the hydrogen atoms.
This unequal charge distribution makes the water molecule extremely "sociable", eager to bond with other water molecules, and gives water its unequaled ability to dissolve compounds.
To analyze how these hydrogen bonds form in small water clusters, Zwier and co-workers used a high-pressure gas expansion to cool water molecules in the gas phase to temperatures as low as 1 degree Kelvin, the equivalent of -457 degrees Fahrenheit.
As the water cooled and condensed into solid clusters, some of the clusters incorporated a single benzene molecule on their surface. The benzene molecule allowed the various clusters to be identified by size using lasers to "weigh" the clusters.
Once he identified the cubic clusters of eight, Zwier and his colleagues applied an infrared laser to excite the clusters, causing the hydrogen bonds in the tiny cubes to stretch and contract. By analyzing the wavelengths of this spectrum, he was able to determine the molecular arrangement of the hydrogen bonds within the cubes.
He found that the cubes were identical in mass and structure, with each cube made up of four molecules of water stacked on top of the other four molecules. Though the hydrogen bonds in the top layer of each cube were oriented in the same manner, the hydrogen bonds in the bottom layers of the cubes took one of two possible arrangements, with the bonds facing either the same direction or opposite direction as the bonds in the top layer of the cube.
"Since the two structures are virtually identical in energy, the orientation that a particular cluster takes depends on the specific collisions the cluster undergoes while it is being made," Zwier says.
"It is interesting that already with only eight water molecules, water makes up two
different 'phases' which differ only in the orientations of the hydrogen bonds,"
he says. "This is the beginnings of what we know to be true in the solid phase. Water
has more solid phases -- nine total -- than any other known pure substance because it can
form phases which differ only in the orientations of the hydrogen bonds."
CONTACT: Zwier, (765) 494-5278; e-mail, zwier@chem.purdue.edu
Michael Lipschutz, professor of chemistry who headed the team that discovered the first evidence for a meteoroid stream in 1993, has found a second stream by analyzing a series of meteorites that have crashed to Earth between 1812 and 1992.
"This new stream appears to have deposited meteorites on Earth over two different intervals," Lipschutz says. "Apparently, the Earth intersected the stream's orbit at these points in time, and some of the meteoroids that were traveling in the stream landed on Earth."
He reported his findings in the April issue of the Journal of Geophysical Research-Planets.
Meteorites are the fragments of small objects called meteoroids that survive passage through the atmosphere and fall on the Earth's surface. Theoretically, a meteoroid stream is made up of a group of rocky fragments that are derived from the breakup of a near-Earth object and then travel in space in the same general orbit, Lipschutz says.
"Meteoroids traveling together would likely represent fragments of the same asteroid, thus they would have a similar chemical makeup," he says.
Using this knowledge, Lipschutz was able to link 17 meteorites that fell to Earth in two separate arrays by analyzing the trace elements in the meteorites. Trace elements are chemical markers that are found in very tiny amounts, such as parts per million or parts per billion.
He then compared the contents of the samples with a set of 33 meteorites of similar composition that fell to Earth at random between 1773 and 1970. The 17 meteorites proved to have a chemical makeup that was similar to each other and significantly different from the meteorites in the random falls.
"Only meteorites from a single source could account for these differences," Lipschutz says.
The 17 meteorites fell to Earth in over a period of time in two separate arrays, indicating that the Earth may have intersected two different parts of the stream, Lipschutz says. The first group of meteorites landed from 1812 through 1831, and a second group of meteorites landed from 1843 and 1992. The falls all occurred during the months of September and October.
In addition, several samples from the stream have interesting histories, Lipschutz says.
"The first meteorite fall, called Borodino, fell two days before the famous 1812 battle there, the ultimate result of which was the devastating retreat of Napoleon's army from Russia," he says, "although no mention appears in history treatises of the Napoleonic era."
The most recent fall, which occurred on Oct. 9, 1992, in Peekskill, N.Y., hit a car and was observed and videotaped over a five-state area, Lipschutz says.
The existence of meteoroid streams was first proposed by Lipschutz and colleagues
in 1986 to explain chemical differences, such as concentrations of trace elements,
between Antarctic and non-Antarctic meteorites.
CONTACT: Lipschutz, (765) 494-5326; e-mail, rnapuml@vm.cc.purdue.edu
"Kids who come from highly noisy or chaotic homes experience less cognitive growth, delayed language skills, have trouble mastering their environments and have increased anxiety," says Theodore Wachs.
Wachs studies environmental influences on early childhood development. He helped create a questionnaire for parents to fill out to measure the level of physical disorganization in the home. The "chaos" questionnaire assesses what he calls "the noise confusion of the home."
He says a chaotic home is one factor associated with adjustment problems in children. For example, in a study of preschool children's reaction to caregiver turnover in day care centers, those from more disorganized homes had more trouble adapting and functioning during the time of change.
"The effects vary with the temperament and sex of the child," he says. "Those who have the most trouble associated with a chaotic home life are boys who are intense, fussy or negative."
Wachs offers these suggestions for toning down "noise confusion" in the home:

|
"Corn growers everywhere will find this program a tremendous help, particularly when diagnosing problems in the field," said Paul Carter, agronomy support manager for Pioneer. "The program has been put together with great care and attention to detail to address early corn growing problems regardless of geography, so it can be used virtually anywhere, by anyone."
"Corn Growth, Development and Diagnostics -- Germination to Knee High" features a diagnostics key that allows the user to match symptoms to pictures within the program. By doing so, the user is guided to possible causes and appropriate solutions.
The program also includes crop management tools such as economic threshold tables for pests and replant decision-making tools. Using the program, farmers will be able to make their own on-the-spot diagnosis. Then, they can work with their agronomist, chemical representative or Extension educator to confirm the diagnosis and find solutions.
Developed under the direction of Lee Schweitzer, professor of agronomy at Purdue, the program began as a teaching tool. Students who used the CD-ROM for classroom exercises showed at least 10 percent to 15 percent improvement -- a letter grade or more -- on quizzes over 87 corn problems, he said.
The CD-ROM requires a minimum 486 processor with 8 MB of RAM and one of the following operating systems: Windows 95/Window NT or Windows 3.1. A Pentium processor with 16 MB or more RAM is recommended for optimum performance. A Power Maintosh version also is available.
"Corn Growth, Development and Diagnostics -- Germination to Knee High" is available
for $80 through the Purdue University Media Distribution Center, 301 S. Second St.,
Lafayette, Ind. 47901-1232; phone (888) 398-4636.
CONTACTS: Schweitzer, (765) 494-4789; e-mail, lschweitzer@dept.agry.purdue.edu;
Pat Arthur, Pioneer, (515) 334-6908.
Because virtually all of today's information technology is computer-driven, there is a huge demand for computer software engineers in just about any field a college-bound high school student can name.
"A computer science degree will make you highly marketable no matter what your field of interest may be," says Wayne Dyksen, associate department head for Purdue University's Department of Computer Sciences. "Computer programming skills are critical not just in the traditional industries like aerospace and telecommunications, but also in finance, medicine, and law."
Last year, 150 companies came to Purdue's West Lafayette campus in search of qualified applicants with computer-related degrees, and his department had just 50 signed up for interviews through the university's placement center.
"Many of these firms were looking to fill dozens and in some cases even hundreds of positions," Dyksen says. "There are far more jobs than there are graduates. Computer scientists can choose the type of work they would like to do and the place they'd like to do it."
The same is true for the two other Purdue schools that offer computer-related degrees; the Schools of Engineering and the School of Technology. Placement rates at for all three Purdue programs are averaging better than 97 percent, and several disciplines boasted 100 percent placement this past year.
U.S. Bureau of Labor Statistics data show that systems analysts and computer engineers are among the fastest growing occupations in the country. In 1994, approximately 678,000 people were employed in these fields. By the year 2005, the number is expected to reach 1.3 million. Demand for computer programmers will also continue to increase, with 601,000 in the work force by 2005.
Dyksen also has noticed a trend in hiring practices that favors new college graduates.
"It used to be that firms were only interested in people who had five years of practical experience in addition to a degree," Dyksen says. "Now they are discovering that they can't find those people, and in many cases the recent college graduates have more up-to-date skills."
Average starting salaries range from the low 30s to the low 40s depending on the industry and the location, but Purdue Computer Science Department graduates generally average about $10,000 more than that.
Purdue was the first university in the country to offer a complete undergraduate curriculum in computer science as well as a doctoral program, and the school is an internationally recognized leader in the field.
While Dyksen would agree that 1997 is the ideal year to be graduating with a computer science degree, 1998 and beyond don't look too bad either.
"The opportunities are really starting to appear limitless," Dyksen says. "People
are no longer asking 'What can I do with a computer science degree?' An easier question
to answer is 'What can't I do?'"
CONTACT: Dyksen, (765) 494-6182; e-mail, wrd@cs.purdue.edu
Compiled by Amanda Siegfried, (765) 494-4709; e-mail, amanda_siegfried@purdue.edu
Purdue News Service: (765) 494-2096; e-mail, purduenews@purdue.edu
Photo Captions
Color photo, electronic transmission, and Web and ftp download available. Photo ID:
Zwier/water
Download here.
Purdue University's "Corn Growth, Development and Diagnostics -- Germination to Knee High" CD-ROM packs bushels of corn and crop problem information and pictures onto one disk. Useful for corn farmers and crop consultants, the multimedia program also has proven its worth in the classroom. (Design by Sharon Katz and Russ Merzdorf)
Color photo, electronic transmission, and Web and ftp download available. Photo ID:
Schweitzer/CD
Download here.