January 21, 2022
Fat’s unexpected role in muscle stem cell fate
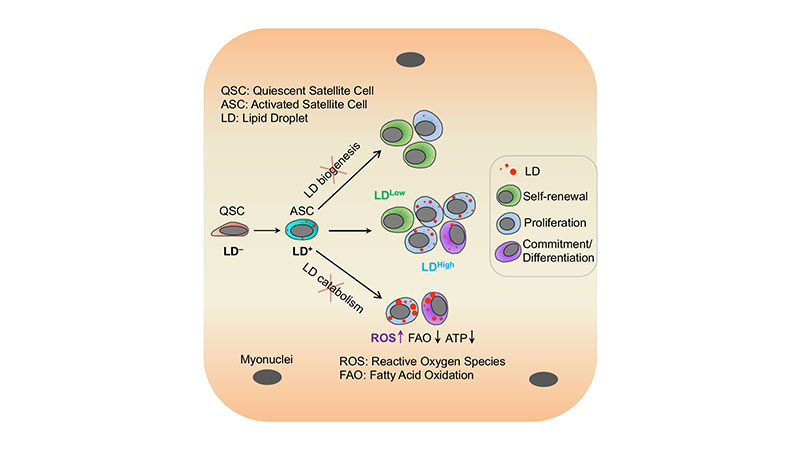 Satellite cells differentiate into muscle cells or self-renew depending on the level of lipid droplets in the cell. Shihuan Kuang, a Purdue University professor of animal sciences, showed for the first time that fat inside adult muscle stem cells regulates their fate. (Purdue University image/courtesy of Shihuan Kuang)
Download image
Satellite cells differentiate into muscle cells or self-renew depending on the level of lipid droplets in the cell. Shihuan Kuang, a Purdue University professor of animal sciences, showed for the first time that fat inside adult muscle stem cells regulates their fate. (Purdue University image/courtesy of Shihuan Kuang)
Download image
WEST LAFAYETTE, Ind. — Scientists have shown for the first time that fat inside adult muscle stem cells regulates their fate.
“No one had seen such dynamics of lipid droplets in these muscle stem cells, so this discovery is very exciting,” said Shihuan Kuang, a professor of animal sciences at Purdue University, who led the team of scientists. “To then find that they play such a strong role in the fate of the stem cells is remarkable. It has potential implications for muscular diseases, aging and animal sciences.”
Cells contain various kinds of fat, or lipids, that are essential for energy production, cell membrane composition and chemical signaling. Special structures, called lipid droplets, safely store this cellular fat.
Rather than existing as a static pool of resources, researchers discovered the number of these droplets changes significantly in an individual cell and varies from cell to cell. The number of the droplets also regulates what the stem cells become.
The discovery, coupled with newly identified roles of lipids in other stem cell types – including cancer stem cells - suggest fat may be involved in much more than previously thought, Kuang said. The findings are detailed in a paper in the journal Cell Reports.
 Shihuan Kuang
Download image
Shihuan Kuang
Download image
The Purdue team studied satellite cells, a stem cell population responsible for muscle development, growth and regeneration. In the adult muscle, these cells are maintained in a dormant state until there is an injury and they are called into action. They then reproduce through divisions and some divided cells become muscle cells to replace the damaged cells, in a process called differentiation. Others return to dormant status through a process called self-renewal.
“Our study showed that stem cells with higher numbers of lipid droplets continued to divide or went on to differentiate and become muscle cells, and those with lower numbers returned to replenish the stock of dormant stem cells for the next injury,” Kuang said. “In fact, during self-renewal, they somehow deplete or rid themselves of the lipid droplets, and in a dormant state contain none.”
They discovered that upon activation, the droplets appear, and as each cell divides, the lipid droplets are not always equally distributed. Some of the descendant cells from division have more droplets than others, and this asymmetric distribution leads to cell fate segregation into self-renewal or differentiation.
“These lipid droplet dynamics are critical to maintaining a healthy balance of the cells,” Kuang said. “We need new muscle cells for the repair, but we don’t want the stem cells dividing uncontrollably, as is what occurs in cancer. The depletion of lipid droplets is like a brake to stop uncontrolled proliferation.”
In previous studies, Kuang’s research team focused on both muscle and fat cells.
“Because we also study fat cells called adipocytes, we had the tools in place to make this discovery,” he said. “During some routine cell staining, Feng Yue, a postdoctoral researcher on our team, noticed the dynamics of lipid droplets in the stem cells. This was surprising because they were not known to be so abundant and dynamic in these cells, at that time. We thought ‘Why are they here?’ We had to find out.”
The team used skeletal muscle stem cells in culture and a mouse model to determine the function of the lipid droplets. The team inhibited the formation of the lipid droplets and then inhibited their utilization to see how these changes would impact cell function.
“The results were amazing as either too many or too few lipid droplets disrupted stem cell fate homeostasis,” said Yue, who is now an assistant professor at the University of Florida. “This suggests that in the future we may be able to stimulate stem cell’s regenerative function by manipulating lipid droplet dynamics in the satellite cells.”
Team members and co-authors also include Stephanie N. Oprescu, a graduate student in the Department of Biological Sciences; Jiamin Qiu, Lijie Gu, Lijia Zhang and Jingjuan Chen, graduate students in animal sciences; Naagarajan Narayanan, a postdoctoral researcher in agricultural and biological engineering; and Meng Deng, assistant professor of agricultural and biological engineering.
Kuang plans to further study the role of the lipid droplets in muscle repair.
“We don’t yet fully understand the upstream regulators and downstream mediators of lipid droplets in the muscle stem cells,” he said. “It may be that there are secondary metabolites formed from degradation of the lipid droplets that also are important. These fats may be much more than an energy source for the cell.”
The National Institutes of Health (NIH-R01AR071649; R03AR068108; F31AR077424; P30CA023168; S10DO20029), Muscular Dystrophy Association (MDA516161) and the U.S. Department of Agriculture (NC1184) funded this work.
Writer: Elizabeth K. Gardner; 765-441-2024; ekgardner@purdue.edu
Source: Shihuan Kuang; 765-494-8283; skuang@purdue.edu
ABSTRACT
Lipid droplet dynamics regulate adult muscle stem cell fate
Feng Yue, Stephanie N. Oprescu, Jiamin Qiu, Lijie Gu, Lijia Zhang, Jingjuan Chen, Naagarajan Narayanan, Meng Deng, and Shihuan Kuang
LINK TO PAPER:
The lipid droplet (LD) is a central hub for fatty acid metabolism in cells. Here we define the dynamics and explore the role of LDs in skeletal muscle satellite cells (SCs), a stem cell population responsible for muscle regeneration. In newly divided SCs, LDs are unequally distributed in sister cells exhibiting asymmetric cell fates, as the LDLow cell self-renews while the LDHigh cell commits to differentiation. When transplanted into regenerating muscles, LDLow cells outperform LDHigh cells in self-renewal and regeneration in vivo. The pharmacological inhibition of LD biogenesis or genetic inhibition of LD catabolism through knockout of Pnpla2 gene (encoding ATGL, the rate-limiting enzyme for lipolysis) disrupts cell fate homeostasis and impairs the regenerative capacity of SCs. Dysfunction of Pnpla2-null SCs is associated with energy insufficiency and oxidative stress that can be partially rescued by antioxidant (N-acetylcysteine) treatment. These results establish a direct link between LD dynamics and stem cell fate determination.
Agricultural Communications: 765-494-8415;
Maureen Manier, Department Head, mmanier@purdue.edu

