Drug Discovery
at Purdue 2016-2017
Drug Discovery
at Purdue 2016-2017


Table of Contents
Introduction
The drug discovery process at Purdue University begins at the nano level and ends with a viable human therapy that can reduce mortality and morbidity of disease. There is a considerable effort in enhancing our portfolio of novel and innovative drug candidates to treat chronic and acute illnesses.
Our researchers are also invested in various approaches to drug discovery, which include understanding of drug targets for future drug therapies, detection technology that will aid clinicians in early diagnosis or monitoring of therapies and design and delivery of drugs.
Researchers associated with the Institute for Drug Discovery are affiliated with colleges from all across campus including Pharmacy, Science, Nutrition, Agriculture, Engineering and Veterinary Medicine.

Purdue University's Institute of Drug Discovery was completed in 2014 and is located at 720 Clinic Drive West Lafayette, Indiana 47907.
Our Drug Discovery facility promotes the discovery, design and development of new drugs through innovative architecture that encourages collaborations in chemistry, medicinal chemistry and biology. The structure accommodates 90 multidisciplinary researchers with 9 faculty offices as well as several conference rooms and common eating facilities. Conference rooms are equipped with videoconferencing capabilities that enable research teams from across the world to interact as though they were present at Purdue. The building provides facilities for organic synthesis, cell culture, analytical chemistry, molecule purification, biochemistry, molecular biology and fluorescent imaging. Core facilities located within the building include the high-throughput screening & chemical genomics, NMR, and mass spectrometry facilities.
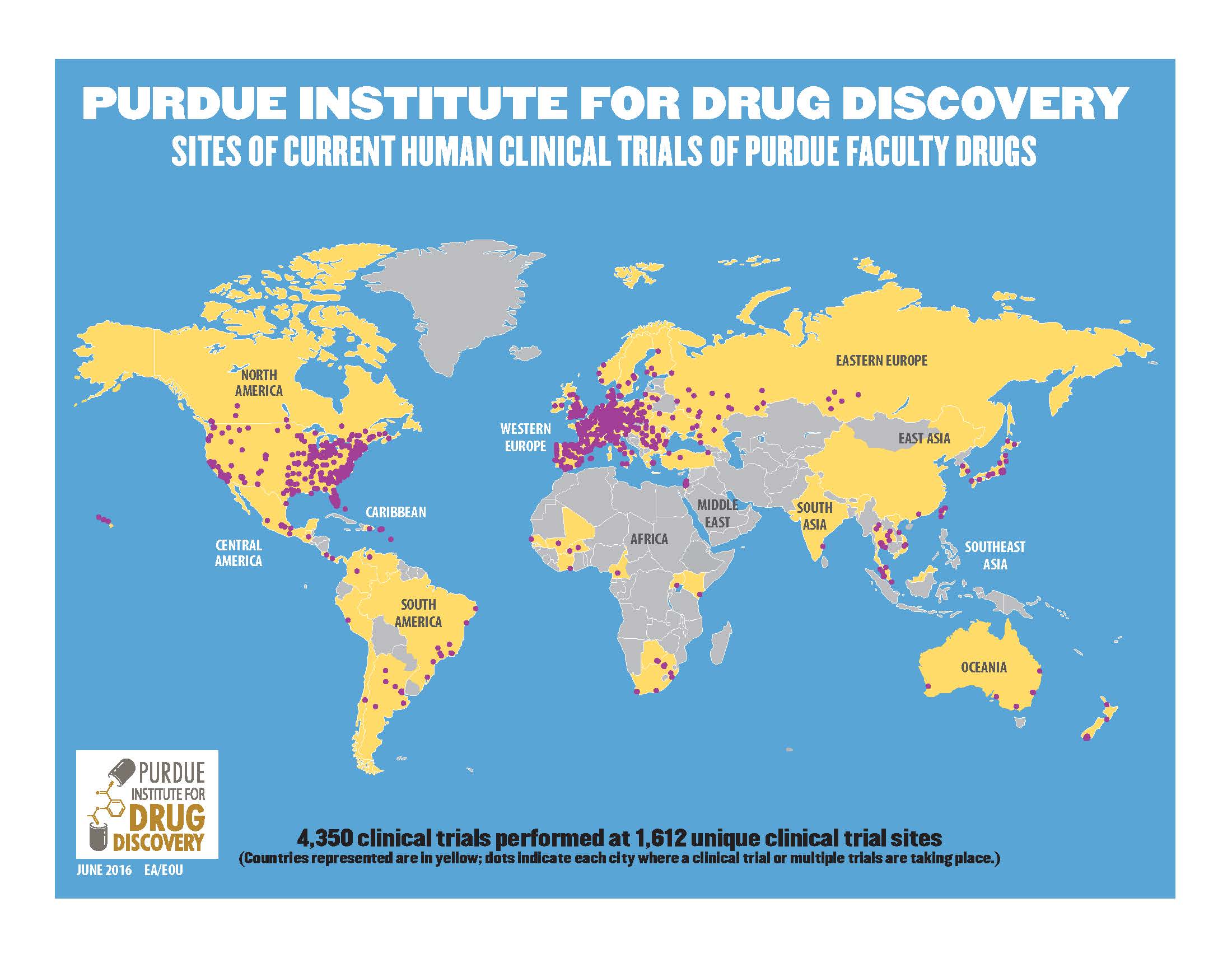
The Purdue Institute of Drug Discovery has broad reaching actions with 4,350 human clinical trials performed with Purdue faculty drugs at 1,612 unique clinical trial sites around the world.
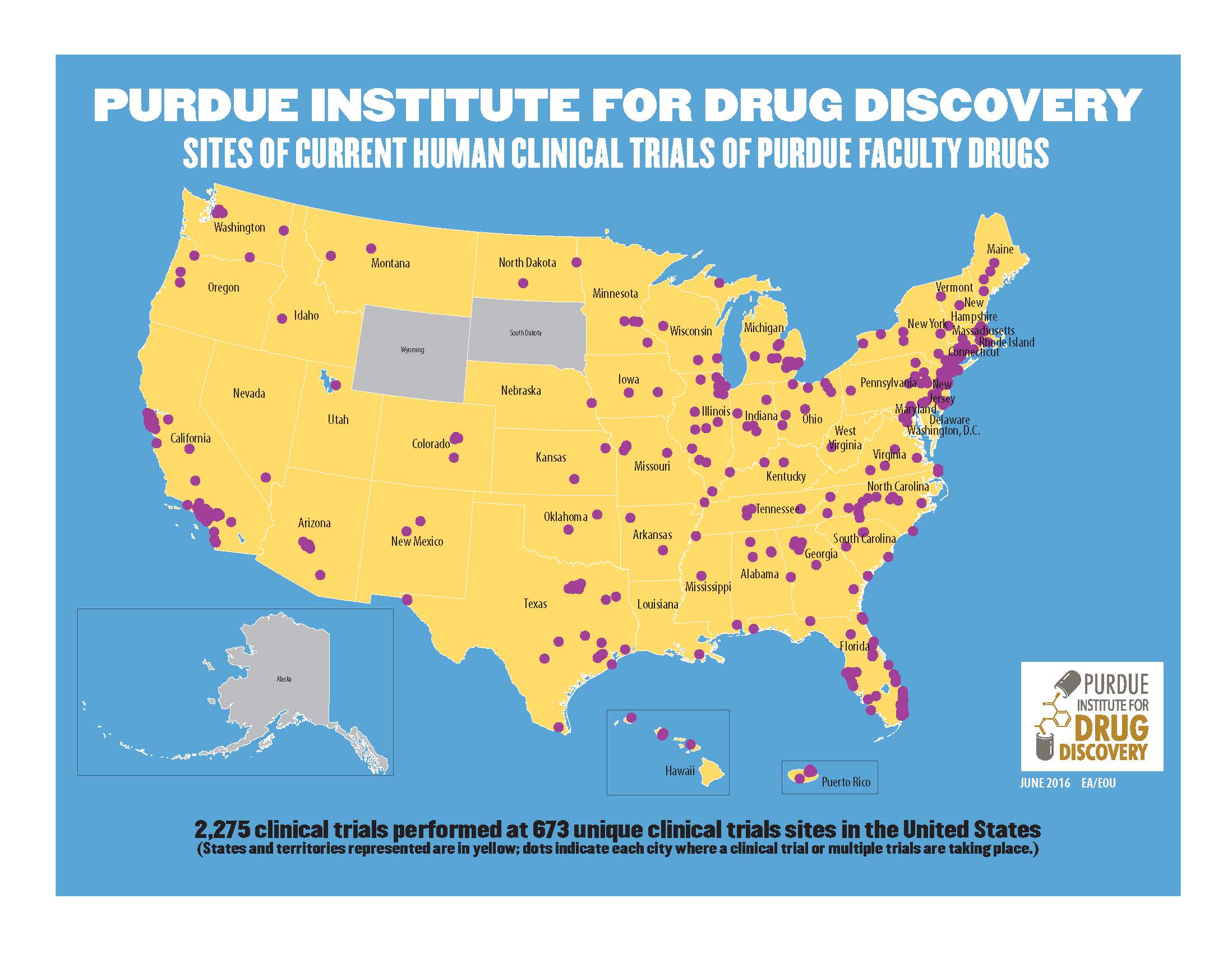
Forty-seven states and Puerto Rico are sites of 2,275 human clinical trials performed with Purdue faculty drugs around the United States at 673 unique sites.
Compounds in Clinical Development
Currently, our researchers are working on several classes of compounds and novel approaches to drug design. The figure below summarizes the active stages of drug discovery.

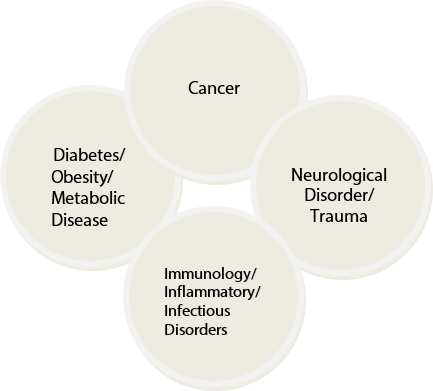
We have 78 faculty researching cancer, 26 faculty researching Diabetes, obesity and metabolic disease, 44 researching immunology, inflammatory and infectious disease, 36 researching neurological disorder/trauma and 19 researching other diseases.
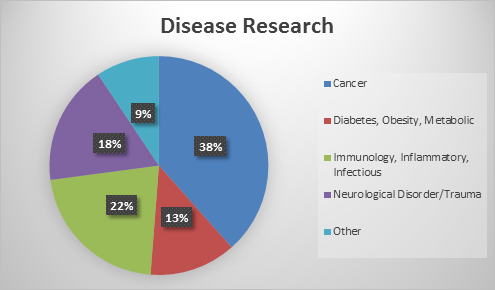
Thirty-eight percent of our faculty associated with the Institute for Drug Discovery are researching cancer, 22% are researching immunology, inflammatory and infectious disease, 18% are researching neurological disorder/trauma, 13% are researching diabetes, obesity and metabolic disease and 9% are researching other diseases.
The Drug Discovery facility houses four pieces of equipment for shared usage with researchers across campus.
NMR Spectrometer

HTS

Mass Spectrometer

Flow Cytometer

Four research buildings on the Purdue West Lafayette campus
directly support our drug discovery mission.
Drug Discovery Facility

Burton D. Morgan Center for Entrepreneurship

Arthur G. Hansen Life Sciences Research Building

Bindley Bioscience Center

Alphabetical List of Drug Discovery Researchers
|
Diagnostics
Target Discovery & Characterization
Drug Synthesis/Optimization
Delivery/Formulations
In Vivo
ADME/DMPK/Tox
Other
Cancer
Diabetes/Obesity/Metabolic Disease
Immunology/Inflammatory/Infectious Disease
Neurological Disorder/Trauma
Other
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CATEGORY | DISEASE(S) | ||||||||||||
| Name | Department | Diagnostics | Target Discovery & Characterization | Drug Synthesis / Optimization | Delivery / Formulations | In Vivo | ADME DMPK Tox | Other | Cancer | Diabetes / Obesity / Metabolic Disease | Immunology / Inflammatory / Infectious Disease | Neurological Disorder / Trauma | Other |
| Aguilar, R. Claudio | Biological Sciences | X | X | X | |||||||||
| Altman, Ryan | Medicinal Chem/Molecular Pharmacology | X | X | X | X | X | |||||||
| Andrisani, Ourania | Basic Medical Sciences | X | X | ||||||||||
| Axelrod, Abram | Chemistry | X | X | X | X | X | |||||||
| Bao, Xiaoping | Chemical Engineering | X | X | X | X | X | X | ||||||
| Barker, Eric | College Of Pharmacy | X | X | X | |||||||||
| Bhunia, Arun | Department Of Food Science | X | X | X | X | ||||||||
| Bowman, Keith | Materials Engineering | X | X | ||||||||||
| Briggs, Scott | Biochemistry | X | X | ||||||||||
| Buhman, Kimberly | Nutrition Science | X | X | X | X | ||||||||
| Byrn, Stephen | Industrial And Molecular Pharmaceutics | X | X | ||||||||||
| Cabot, Ryan | Animal Sciences | X | X | X | X | ||||||||
| Camarillo, Ignacio | Biological Sciences | X | X | ||||||||||
| Childress, Michael | Veterinary Clinical Sciences | X | X | ||||||||||
| Chmielewski, Jean | Chemistry | X | X | X | X | X | X | X | |||||
| Chopra, Gaurav | Chemistry | X | X | X | X | X | |||||||
| Cooks, Graham | Chemistry | X | X | ||||||||||
| Cushman, Mark | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Das, Chittaranjan | Chemistry | X | |||||||||||
| Davisson, Vincent | Medicinal Chem/Molecular Pharmacology | X | X | ||||||||||
| Dykhuizen, Emily | Medicinal Chem/Molecular Pharmacology | X | X | ||||||||||
| Figueiredo, Marxa | Basic Medical Sciences | X | X | X | X | X | |||||||
| Flaherty, Daniel | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Fortin, Jessica | Basic Medical Sciences | X | X | X | |||||||||
| Foster, David | Department Of Pharmacy Practice | X | X | X | X | ||||||||
| Freeman, Jennifer | Health Sciences | X | X | X | |||||||||
| Garcia, R Edwin | Materials Engineering | X | X | X | |||||||||
| Ghosh, Arun | Chemistry | X | X | X | X | X | |||||||
| Gore, Jay | Mechanical Engineering | X | X | X | |||||||||
| Hall, Mark | Biochemistry | X | X | ||||||||||
| Hazbun, Tony | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Hill, Catherine | Entomology | X | X | X | X | ||||||||
| Hockerman, Gregory | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Hogan, Daniel | Veterinary Clinical Sciences | X | X | X | X | X | X | ||||||
| Hogenesch, Harm | Comparative Pathobiology | X | X | ||||||||||
| Hrycyna, Christine | Chemistry | X | X | ||||||||||
| Hu, Chang-Deng | Medicinal Chem/Molecular Pharmacology | X | X | ||||||||||
| Huang, Rong | Medicinal Chem/Molecular Pharmacology | X | X | X | X | X | X | ||||||
| Hudmon, Andy | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Jeong, Hyun-Young | Industrial And Physical Pharmacy | X | X | X | |||||||||
| Jiang, Qing | Nutrition Science | X | X | X | X | X | |||||||
| Jiang, Wen | Biological Sciences | X | X | X | X | X | |||||||
| Kasinski, Andrea | Biological Sciences | X | X | X | X | ||||||||
| Kihara, Daisuke | Biological Sciences/Computer Science | X | X | X | |||||||||
| Kim, Kee Hong | Department Of Food Science | X | X | X | X | ||||||||
| Kirchmaier, Ann | Biochemistry | X | X | ||||||||||
| Knapp, Deborah | Veterinary Clinical Sciences | X | X | ||||||||||
| Knipp, Gregory | Industrial And Molecular Pharmaceutics | X | X | X | X | X | |||||||
| Ko, Jeff | Veterinary Clinical Sciences | X | X | X | |||||||||
| Krusemark, Casey | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Kuang, Shihuan | Animal Sciences | X | X | X | X | X | |||||||
| Kuhn, Richard | Biological Sciences | X | X | X | |||||||||
| Lelievre, Sophie | Basic Medical Sciences | X | X | ||||||||||
| Leung, Yuk Fai | Biological Sciences | X | X | X | X | X | |||||||
| Liceaga, Andrea | Department Of Food Science | X | X | ||||||||||
| Lim, Seung-Oe | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Lipton, Mark | Chemistry | X | X | X | X | ||||||||
| Liu, Julie | Chemical Engineering | X | X | ||||||||||
| Liu, Shuang | Health Sciences | X | X | X | X | ||||||||
| Liu, Xing | Biochemistry | X | X | X | |||||||||
| Low, Philip | Chemistry | X | X | X | X | X | X | X | |||||
| Luo, Zhao-Qing | Biological Sciences | X | X | X | |||||||||
| Lyon, Angeline | Chemistry | X | X | X | X | ||||||||
| Matosevic, Sandro | Industrial And Physical Pharmacy | X | X | X | |||||||||
| Mattoo, Seema | Biological Sciences | X | X | X | |||||||||
| Mesecar, Andrew | Purdue Center For Cancer Research | X | X | X | |||||||||
| Mittal, Suresh | Comparative Pathobiology | X | X | X | X | ||||||||
| Mohammed, Sulma | Comparative Pathobiology | X | X | X | |||||||||
| Nagy, Zoltan | Chemical Engineering | X | X | X | X | X | X | ||||||
| O'Brien, Valerie | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Overholser, Brian | Department Of Pharmacy Practice | X | X | ||||||||||
| Park, Chiwook | Medicinal Chem/Molecular Pharmacology | X | X | X | X | X | |||||||
| Park, Kinam | Biomedical Engineering | X | X | X | X | ||||||||
| Parkinson, Elizabeth | Chemistry | X | X | X | X | ||||||||
| Paschou, Peristera | Biological Sciences | X | X | X | |||||||||
| Pienaar, Elsje | Biomedical Engineering | X | X | X | X | X | |||||||
| Pinal, Rodolfo | Industrial And Molecular Pharmaceutics | X | X | X | X | ||||||||
| Porterfield, D. Marshall | Agricultural And Biological Engineering | X | X | X | X | ||||||||
| Post, Carol | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Ramachandran, Padinjaremadhom | Chemistry | X | X | X | |||||||||
| Ramkrishna, Doraiswami | Chemical Engineering | X | X | ||||||||||
| Ratliff, Timothy | Comparative Pathobiology | X | X | X | |||||||||
| Reklaitis, Gintaras | Chemical Engineering | X | X | X | X | X | |||||||
| Rice, Christopher | Comparative Pathobiology | X | X | X | X | X | X | ||||||
| Rochet, Jean-Christophe | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Rossie, Sandra | Biochemistry | X | X | ||||||||||
| Savaiano, Dennis | Nutrition Science | X | X | ||||||||||
| Savran, Cagri | Mechanical Engineering | ||||||||||||
| Shah, Kavita | Chemistry | X | X | ||||||||||
| Shi, Riyi | Basic Medical Sciences | X | X | ||||||||||
| Simpson, Garth | Chemistry | X | X | X | X | X | |||||||
| Sintim, Herman | Chemistry | X | X | X | X | ||||||||
| Smith, Daniel | Industrial And Molecular Pharmaceutics | X | X | X | X | X | X | ||||||
| Sowinski, Kevin | Department Of Pharmacy Practice | X | X | ||||||||||
| Stahelin, Robert | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Stauffacher, Cynthia | Biological Sciences | X | |||||||||||
| Tao, Andy | Computer Science | X | X | X | |||||||||
| Taylor, Lynne | Industrial And Molecular Pharmaceutics | X | X | X | X | X | |||||||
| Tesmer, John | Biological Sciences | X | X | X | X | X | X | ||||||
| Thompson, David H. | Chemistry | X | X | X | |||||||||
| Tisdale, James | Department Of Pharmacy Practice | X | X | ||||||||||
| Topp, Elizabeth | Industrial And Physical Pharmacy | X | X | X | X | ||||||||
| Trader, Darci | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Uyeda, Christopher | Chemistry | X | X | X | |||||||||
| Vanhaezebrouck, Isabelle | Veterinary Clinical Sciences | X | X | ||||||||||
| Verma, Mohit | Agricultural And Biological Engineering | X | X | X | X | ||||||||
| Watts, Val | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Wei, Alexander | Chemistry | X | X | X | X | ||||||||
| Wendt, Michael | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Wilker, Jonathan | Chemistry | X | X | ||||||||||
| Wirth, Mary | Chemistry | X | X | ||||||||||
| Yang, Yang | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Yang, Danzhou | Medicinal Chem/Molecular Pharmacology | X | X | X | |||||||||
| Yao, Yuan | Department Of Food Science | X | X | X | X | ||||||||
| Yeo, Yoon | Industrial And Physical Pharmacy | X | X | X | |||||||||
| Yuan, Chongli | Chemical Engineering | X | X | X | X | ||||||||
| Zhang, Guangjun | Comparative Pathobiology | X | |||||||||||
| Zhang, Zhong-Yin | Medicinal Chem/Molecular Pharmacology | X | X | X | X | ||||||||
| Zheng, Wei | Health Sciences | X | X | ||||||||||
| Zhou, Daoguo | Biological Sciences | X | X | ||||||||||
| Zhou, Qi | Industrial And Physical Pharmacy | X | X | X | |||||||||
| , | |||||||||||||
Diagnostics
Diagnostics

Abram Axelrod
Chemistry
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Eric Barker
College of Pharmacy
College Of Pharmacy
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Proper control of the chemical mediators of neurotransmission requires dynamic regulation of neurotransmitter concentrations in the synapse. For most transmitters such as serotonin, clearance from the synapse is mostly dependent upon an active uptake system mediated by Na+ and Cl--dependent transporter proteins located on presynaptic terminals. In addition to these active transport systems, recent evidence suggests that certain neuromodulatory substances such as the putative endogenous cannabinoid anandamide are removed from the synapse by facilitative transport processes. Our research focuses on identifying structural determinants of functional and pharmacological properties of serotonin and anandamide transporters. These studies use multiple techniques including expression and characterization of cloned transporters in mammalian cells, electrophysiology, immunoblotting, the formation of chimeric proteins, and site-directed mutagenesis to investigate the molecular properties of these transporters.
Serotonin transporters (SERTs) are of particular clinical interest because they are the molecular targets for many antidepressants such as imipramine (Tofranil), sertraline (Zoloft), and fluoxetine (Prozac), as well as many drugs of abuse like cocaine and amphetamine. The cloning of SERT revealed a proposed protein structure consisting of 12 transmembrane-spanning domains. The question related to this structure is what amino acids are involved in the formation of the binding site for SERT inhibitors and substrates? We are currently using chimeric protein and mutagenesis strategies to identify amino acids involved in the pharmacological properties of cocaine and amphetamines like MDMA or "ecstasy." In addition to molecular biology approaches, we anticipate using structure-activity relationship studies and molecular modeling to further refine our understanding of drug binding and action at serotonin transporters.
We are also interested in the identification and characterization of transport proteins for the endogenous cannabinoid anandamide. Anandamide (N-arachidonylethanolamide) is a member of a larger class of fatty acid derived signaling molecules that possess in vivo and in vitro marijuana-like actions. Evidence suggests that anandamide is rapidly transported into neurons and astrocytes after release, where it undergoes rapid intracellular degradation. Anandamide uptake appears to be a facilitative process, and we have evidence that the intracellular metabolizing enzyme, fatty acid amide hydrolase (FAAH), plays an important role in maintaining the inward gradient needed for anandamide transport. Future studies in this area will focus on better understanding the role of FAAH in anandamide uptake as well as identifying novel proteins that may also be involved with anandamide transport.
Arun Bhunia
College of Agriculture/College of Veterinary Medicine
Department Of Food Science
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Pathogen and Toxin Detection:
- Biosensor technologies including laser light scattering, mammalian cell-based, and fiber optic sensors for rapid and high throughput screening of live pathogens and toxins in food
- Development of biorecognition including antibodies, receptors, ligands, and microbiological growth media
Host-Pathogen Interaction and Control Strategies Using Probiotics:
- Understanding the molecular and cellular mechanism of Listeria monocytogenes colonization and translocation through epithelial barrier during intestinal phase of infection
- Prevention and control using bioengineered probiotic and antimicrobial peptide loaded biocompatible nano-carrier
Graham Cooks
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
We are interested in the use of mass spectrometry (MS) to identify markers for diseases such as prostate cancer. We are particularly interested in tissue imaging using MS to supplement standard histological methods. These experiments are best conducted on site, during surgery, and our attempts at building high-performance handheld mass spectrometers are consistent with this aim.
Desorption electrospray ionization (DESI) is a new MS ionization method that is applicable in the ambient environment. We are interested in extending its use to problems of in situ disease diagnosis as well as clinical analysis.
David Foster
College of Pharmacy
Department Of Pharmacy Practice
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
My research interests are focused on the study of alterations in drug and nutrient disposition and drug effects in critically ill patients. Current research includes evaluation of changes in intestinal permeability to xenobiotics in critical illness. Specifically, this research involves the investigation of alterations in drug and nutrient absorption by passive and active transport mechanisms, and the molecular mediators underlying these changes in burn injury and sepsis. A related area of research is the use of natural anti-inflammatory compounds to attenuate inflammation-related changes in intestinal function. Other interests include the study of the contribution of active transport processes to variability in drug disposition in a number of patient populations. Dr. Foster's clinical interests are focused the provision of pharmacotherapy to critically-ill patients, with an emphasis on burn and trauma patients.
Jay Gore
College of Engineering
Mechanical Engineering
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Sensors and models of fluid flow and chemical reaction processes in metabolic and other biological activities
Daniel Hogan
Veterinary Clinical Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac, vascular, thrombosis, valvular
Molecular/Cellular Target(s)
TGF-B and valvulopathy
Research Interest and Expertise
Our research focuses on cardiovascular therapeutics, including heart failure and antithrombotics. We have expertise in veterinary clinical trials, pre-clinical animal trials and animal modeling.
Casey Krusemark
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Cancer
Molecular/Cellular Target(s)
- Protein Kinases (broadly)
- chromodomains
Research Interest and Expertise
Our work centers on the use of DNA-encoding approaches for discovery and development of biologically active small molecules. In one area, we utilize DNA-programmed combinatorial chemistry to construct novel chemical libraries of DNA-encoded small molecules. We are using these libraries to develop peptidomimetic inhibitors of protein-protein interactions. In a second area, we have developed a DNA-based assay approach for biochemical assays including several enzymatic assays and ligand binding assays. We work to apply these assays in proteomic activity profiling and in small molecule screening.
Our lab has extensive expertise in DNA-encoded chemical approaches and in design of DNA-compatible combinatorial chemical libraries. Additional expertise lies generally in the areas of bioconjugation chemistry, peptide/peptidomimetic synthesis, and DNA sequence analysis.
Julie Liu
College of Engineering
Chemical Engineering
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
My laboratory is focused on engineering modular proteins for applications in tissue engineering, surgical adhesives, and biosensor diagnostic assays. In particular, we have investigated peptide-based cues, such as domains derived from growth factors including bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF), and their subsequent effect on cell behavior. We have evaluated the physical properties of crosslinked hydrogels made from these materials and have investigated human mesenchymal stem cell (hMSC) response to these materials. In addition, we are currently developing biosensors for determining the epigenetic state of live cells. These biosensors would facilitate isolation of cell populations with homogeneous epigenetic modifications and thus enable studies of drugs targeted for specific disease states.
Shuang Liu
College of Health and Human Sciences
Health Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
More than 70 million Americans live with cardiovascular diseases. Accurate diagnosis is highly desirable so that appropriate therapeutic regimens can be given before irreversible damage occurs in the patients with known or suspected coronary artery disease (CAD). Myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) is an integral component in routine clinical evaluation of CAD patients. In spite of recent development of stress echocardiography and coronary CT angiography, SPECT MPI remains the mainstay for noninvasive diagnosis of CAD.
Molecular/Cellular Target(s)
Cardiolipin as the Molecular Target for Diagnosis of Heart Diseases. Heart is one of the organs rich with mitochondria. The mitochondrial density is as high as 40% of the cellular volume in myocytes. It is not surprising that mitochondrion has been a target for development of myocardial perfusion radiotracers that tend to localize inside the mitochondrial matrix. In contrast, CL is embedded in the inner mitochondrial membrane and constitutes up to as high as ~20% of its total lipid content. The fact that CL alterations underlie the myocardial dysfunction makes CL a useful and multifunctional biomarker for cardiovascular diseases (particularly HF), and provides the conceptual basis to develop molecular imaging probes that can be used to measure early CL changes noninvasively in the HF patients and those with diabetes.
Research Interest and Expertise
I worked at DuPont Medical Imaging Division (new Lantheus Medical Imaging Inc.) for nine years, and have research interests include receptor-based target radiopharmaceuticals, new bifunctional chelators, development of new techniques for radiolabeling of small biomolecules, formulation development, design/synthesis/evaluation of metal complexes as MRI contrast agents for cardiac perfusion imaging, and coordination chemistry of radiopharmaceuticals. There have been tremendous research efforts from his research group in the development of novel radiotracers for early tumor detection and diagnosis of cardiovascular diseases. These efforts rely on identification and the use of small biomolecules as “vehicles” to carry a diagnostic radionuclide to the tumor cells. Imaging with radiolabeled small biomolecules allows us to monitor the tumor biological changes at the molecular level. Over the last 10 years, Dr. Liu has become the leader in radiolabeled cyclic RGD peptides as integrin αvβ3-specific SPECT and PET radiotracers for imaging the integrin expression αvβ3 in rapidly growing and metastatic tumors. Dr. Liu is the author or co-author over 160 scientific publications, and has been granted 30 US patents and PCT applications. Dr. Liu’s contributions also have significant impacts on inorganic chemistry, radiochemistry, radiopharmaceutical development, bioconjugates chemistry, molecular imaging, and nuclear medicine. His research has been supported by grants from the National Institute of Health, Department of Energy, American Heart Association, and industry.
Philip Low
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Cancers of the ovaries, prostate, cervix, kidneys, colon, lung, breast
- Autoimmune/Inflammatory diseases such as rheumatoid arthritis, atherosclerosis, pulmonary fibrosis, Crohn’s disease, osteoarthritis, psoriasis
- Influenza, malaria, HIV
- Obesity, diabetes
- Sickle cell disease
Molecular/Cellular Target(s)
Folate receptors (alpha, beta, and delta), carbonic anhydrase IX, CCK2R, prostate specific membrane antigen (PSMA), luteinizing hormone-releasing hormone (LHRH), bombesin receptor, aminopeptidase N, fibroblast activation protein, neuraminidase, red blood cell kinases, band 3
Research Interest and Expertise
To date, we have developed targeted therapeutic and/or imaging agents for a variety of cancers (e.g. ovarian, lung, kidney, endometrial, breast and prostate), several inflammatory diseases (rheumatoid arthritis, Crohn’s disease, osteoarthritis, organ transplant rejection, psoriasis, etc.), diabetes, atherosclerosis and a variety of infectious diseases (e.g. malaria, influenza virus, Staphylococcus, Pseudomonas, etc.). Eleven drugs stemming from research in my lab are currently undergoing human clinical trials (mainly at Endocyte, Inc., HuLow, and On Target Laboratories, three companies that I have founded).
Interests include: Imaging of malignant diseases; isolation and analysis of circulating tumor cells; fluorescence guided surgery using tumor-targeted fluorescent dyes; and personalized medicine, therapies for infectious diseases.
Chiwook Park
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Proteins are dynamic molecules. Even under native conditions, they do not adopt a single static conformation. Rather, they access many different conformations in their native state ensemble. This native state ensemble includes small fluctuations around the native conformation, partially unfolded forms, and even globally unfolded forms. The distribution of these conformations and the kinetic barriers between the conformational states define the conformational energy landscapes of proteins. My research interest is investigating conformational energy landscapes of proteins and deciphering the relationship between the energetics of proteins and their biochemical functions, such as catalysis, signal transduction, and ligand binding. We use proteolysis as a major tool to probe protein structures and dynamics as well as conventional spectroscopic methods. We also use proteomics extensively for investigating energy landscapes of proteins on a system level.
Peristera Paschou
Biological Sciences
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Neurodevelopmental Disorders, Neurogenerative Disorders, Neuromuscular Disorders
Molecular/Cellular Target(s)
Genomic analysis, Neuroimaging analysis
Research Interest and Expertise
Dr Paschou works at the intersection of Data Science and genomics research. She studies human genetic variation around the world aiming to understand the cause of neurological and neuropsychiatric disorders and identify predictive biomarkers. She is an expert in genomewide association studies (GWAS) aiming to elucidate the genetic basis of complex disease and studies population genetic structure around the world. She has a long-standing interest in Tourette Syndrome (TS) and related neurodevelopmental disorders of childhood onset and leads multiple international consortia that are pioneering investigations in this field. She currently coordinates a mega-GWAS for TS and is Chair of the ENIGMA-Tourette Syndrome Working Group, bringing together genetics and neuroimaging datasets from multiple collaborating sites with a goal to understand brain structure and function in TS. She also works on understanding the link between neurodevelopment and neurodegeneration, focusing on the identification of predictive biomarkers for Alzheimer’s Disease and Related Dementias.
D. Marshall Porterfield
College of Engineering
Agricultural And Biological Engineering
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
- Biosensors
- Cell signaling
- Cellular metabolism
- Lab-on-a-chip systems for cell physiology
Christopher Rice
Comparative Pathobiology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Several human and animal diseases caused by various pathogenic free-living amoeba. Acanthamoeba Keratitis (AK) – Acanthamoeba species. Granulomatous Amoebic Encephalitis (GAE) – Acanthamoeba species. Cutaneous skin lesions – Acanthamoeba species. Disseminated Acanthamoeba Infection – Acanthamoeba species.
Primary Amoebic Meningoencephalitis (PAM) – Naegleria fowleri.
Balamuthia Amoebic Encephalitis (BAE) or GAE – Balamuthia mandrillaris.
Cutaneous skin lesions – Balamuthia mandrillaris.
Disseminated Balamuthia Infection – Balamuthia mandrillaris.
Molecular/Cellular Target(s)
Most of our therapeutic targets have been suggested through phenotypic whole cell screening against each of these three pathogenic free-living amoebae ((FLA); Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris). This chemical inference approach identifies compounds which are active against the amoeba and then suggests a specific protein target. Please see https://www.ssgcid.org/ for an up-to-date list of requested targets for structural determination and their progress by our collaborators.
Research Interest and Expertise
My primary focus for the last decade has been identifying essential amino acid biochemical pathways through target and phenotypic based drug discovery on these amoebae. I have developed high-throughput screening methods to assess hundreds-to-thousands of compounds for drug repurposing or as chemical starting points to optimise into potential prophylactic or curative therapeutics in the future. My lab is interested in drug discovery and development for orphan diseases, host-pathogen interactions, understanding the diversity of species and variable pathobiology they may cause, using pathobiological in vivo models to assess disease and develop novel treatments for these devastating diseases.
Alexander Wei
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Cancer, ovarian and bladder (using orthotopic animal models)
- Bacterial infections
Molecular/Cellular Target(s)
- SKOV3 cells
- Tumor-associated macrophages
- Bacillus anthracis
- Chlamydia trachomatis
- Listeria monocytogenes
- Pseudomonas aeruginosa
- Salmonella enterica
- Staphylococcus aureus / MRSA
- Streptococcus pneumoniae
- Yersinia enterocolitica
- Albumin receptors
- Hemin receptors (Isd, etc.)
- Siderophore receptors (FoxA, FhuD2, FhuE)
Research Interest and Expertise
- Targeting ligands for pathogen detection and treatment, with particular interests in respiratory-tract and sexually transmitted infections.
- Targeted photodynamic therapy/inactivation (PDT / PDI) using photoactive hemin derivaties
- Identifying key serum proteins as mediators in nanoparticle tracking and cell uptake
- siRNA uptake and release in ovarian cancer cells
Michael Wendt
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Breast cancer
Molecular/Cellular Target(s)
EGFR, Her2, FGFR
Research Interest and Expertise
Research in the Wendt is focused on the role of epithelial-mesenchymal transition (EMT) in breast cancer metastasis. EMT is associated with resistance to several chemotherapeutic drugs and targeted molecular compounds. Recent studies by the Wendt has identified fibroblast growth factor receptor (FGFR) as major driver of drug resistance, particularly in the metastatic setting. Furthermore, cells that have undergone EMT become preferentially sensitive to inhibition of FGFR kinase activity. Work in the Wendt utilizes 3D cell culture and in vivo disease modeling in combination with an array of small molecule and biological approaches to optimize FGFR targeting for the treatment of metastatic and drug resistant breast cancer.
Mary Wirth
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
We work at the interface of chemistry and medicine, and our focus is to create technology for earlier detection of diseases. The dream of 21st century medicine is that simple lab tests will reveal diseases well before the onset of symptoms, while the disease is easily curable. We are using nanotechnology to modernize the materials used for lab tests and for the discovery of the biomarkers that are the targets of lab tests.
Danzhou Yang
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Molecular/Cellular Target(s)
DNA secondary structures and interactive proteins
Research Interest and Expertise
DNA-targeted anticancer drugs and structure-based rational drug design; Structures and functions of DNA secondary structures as cancer-specific molecular targets; DNA G-quadruplex secondary structures and their interactions with small molecule drugs and proteins; DNA-targeted anticancer drugs that inhibit transcription factors and topoisomerases. High-field NMR macromolecule structure determination.
Chongli Yuan
College of Engineering
Chemical Engineering
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Molecular/Cellular Target(s)
- Develop sensors for monitoring cell response to extracellular matrix, drug and environmental stimuli.
- Develop platforms for detecting drug resistance
Research Interest and Expertise
Our lab is currently focused on studying the effect of epigenetic modifications, i.e., DNA methylation and histone post-translational modifications, on chromatin structure and identifying sequence-specific epigenetic changes as potential early stage biomarkers for cancer and neurological diseases. We also develop novel engineering probes to detect and monitor disease-related epigenetic features as well as various other sensors for disease detection and management.
Target Discovery & Characterization
Target Discovery & Characterization

R. Claudio Aguilar
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Developmental diseases: Lowe syndrome, Oligophrenia
- Neurodegenerative diseases: Huntington disease and Spinocerebelar ataxias
- Bladder cancer
- Invasive cancers
Molecular/Cellular Target(s)
- Endocytic machinery: clathrin, epsin, adaptor proteins (APs)
- Huntingtin, ataxin-3
- Notch and Wnt signaling
- Ocrl1 and Ophn1
Research Interest and Expertise
My laboratory is focused in the study of protein and vesicle trafficking in relation to the processes of cell polarity establishment (a feature that is key for animal development and crucial for physiological functions such as synaptic transmission and immune response) as well as carcinogenic transformation. In order to pursue our research goals we routinely use genetic, biochemistry and cell biology techniques with yeast and mammalian cells. We study protein-protein interactions at molecular level by using bioinformatics, biochemical and genetic tools (like the two-hybrid system) and we investigate the physiological relevance of these interactions by using functional assays, microscopy (of live and fixed cells) and genetic approaches.
Ourania Andrisani
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Liver Cancer
Research Interest and Expertise
My interests and expertise are on molecular mechanisms of transcriptional regulation, epigenetics, and signal transduction involved in cell growth control, cellular differentiation, and cancer pathogenesis.
My laboratory has been studying cellular pathways induced by Hepatitis B virus (HBV) infection that are involved in virus biosynthesis and disease pathogenesis. Our goal is to identify essential mechanisms that can be targeted to suppress HBV infection and the resulting HBV-mediated liver cancer. One such mechanism identified by our studies is activation of the cellular S/T kinase Polo-like-kinase 1 (Plk1) by the virus-encoded oncogenic HBx protein. We have shown that Plk1 activation exerts a crucial role both in HBx-mediated oncogenic transformation, and serves as a positive effector role in HBV replication. Our ongoing studies with the team of Prof. Zoulim M.D.,Ph.D., Medical Co-Director of the Liver Department at Lyon University Hospital, France, support that Plk1 can be explored as a novel antiviral target for the suppression of HBV infection.
In addition, we have shown that Plk1 activation by HBx downregulates the activity of two chromatin modifying complexes, the Polycomb repressive complex2 (PRC2) and the LSD1/CoREST/HDAC1. The consequence of this epigenetic deregulation is re-expression of a hepatic cancer stem cell (hCSC)-like group of genes. In collaboration with the team of Professor Philippe Merle, M.D., Ph.D., Medical Co-Director of the Liver Department at Lyon University Hospital, France, we have shown that expression of this gene signature in clinical samples is associated with poor patient prognosis. Thus, our studies have provided the first direct evidence that HBV epigenetically reprograms normal hepatocytes.
Our current studies have identified yet another essential piece of this epigenetic puzzle, the RNA helicase DDX5, involved both in HBV replication and HBV-associated liver cancer. In collaboration with Dr. E. Tran, Biochemistry Department at Purdue who is an expert in the study of yeast RNA helicases, we are investigating the role and mechanism of the interaction between PRC2 and DDX5, from the point of view of the function/stability of the PRC2 complex. In addition, in collaboration with the teams of Prof. Merle and Zoulim, we will analyze the clinical relevance of our observations, both in terms of HBV biosynthesis and HCC pathogenesis. Thus, our studies, with a strong team of collaborators, will reveal novel insights into HBV-mediated liver cancer, viral infection and regulation of DDX5 activity and function. Importantly, these studies are necessary first steps to elucidate novel targets for therapeutic intervention targeting essential steps for HBV infection and HBV-associated liver cancer.
Abram Axelrod
Chemistry
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Xiaoping Bao
Chemical Engineering
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Eric Barker
College of Pharmacy
College Of Pharmacy
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Proper control of the chemical mediators of neurotransmission requires dynamic regulation of neurotransmitter concentrations in the synapse. For most transmitters such as serotonin, clearance from the synapse is mostly dependent upon an active uptake system mediated by Na+ and Cl--dependent transporter proteins located on presynaptic terminals. In addition to these active transport systems, recent evidence suggests that certain neuromodulatory substances such as the putative endogenous cannabinoid anandamide are removed from the synapse by facilitative transport processes. Our research focuses on identifying structural determinants of functional and pharmacological properties of serotonin and anandamide transporters. These studies use multiple techniques including expression and characterization of cloned transporters in mammalian cells, electrophysiology, immunoblotting, the formation of chimeric proteins, and site-directed mutagenesis to investigate the molecular properties of these transporters.
Serotonin transporters (SERTs) are of particular clinical interest because they are the molecular targets for many antidepressants such as imipramine (Tofranil), sertraline (Zoloft), and fluoxetine (Prozac), as well as many drugs of abuse like cocaine and amphetamine. The cloning of SERT revealed a proposed protein structure consisting of 12 transmembrane-spanning domains. The question related to this structure is what amino acids are involved in the formation of the binding site for SERT inhibitors and substrates? We are currently using chimeric protein and mutagenesis strategies to identify amino acids involved in the pharmacological properties of cocaine and amphetamines like MDMA or "ecstasy." In addition to molecular biology approaches, we anticipate using structure-activity relationship studies and molecular modeling to further refine our understanding of drug binding and action at serotonin transporters.
We are also interested in the identification and characterization of transport proteins for the endogenous cannabinoid anandamide. Anandamide (N-arachidonylethanolamide) is a member of a larger class of fatty acid derived signaling molecules that possess in vivo and in vitro marijuana-like actions. Evidence suggests that anandamide is rapidly transported into neurons and astrocytes after release, where it undergoes rapid intracellular degradation. Anandamide uptake appears to be a facilitative process, and we have evidence that the intracellular metabolizing enzyme, fatty acid amide hydrolase (FAAH), plays an important role in maintaining the inward gradient needed for anandamide transport. Future studies in this area will focus on better understanding the role of FAAH in anandamide uptake as well as identifying novel proteins that may also be involved with anandamide transport.
Arun Bhunia
College of Agriculture/College of Veterinary Medicine
Department Of Food Science
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Pathogen and Toxin Detection:
- Biosensor technologies including laser light scattering, mammalian cell-based, and fiber optic sensors for rapid and high throughput screening of live pathogens and toxins in food
- Development of biorecognition including antibodies, receptors, ligands, and microbiological growth media
Host-Pathogen Interaction and Control Strategies Using Probiotics:
- Understanding the molecular and cellular mechanism of Listeria monocytogenes colonization and translocation through epithelial barrier during intestinal phase of infection
- Prevention and control using bioengineered probiotic and antimicrobial peptide loaded biocompatible nano-carrier
Scott Briggs
College of Agriculture
Biochemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Several histone methyltransferases and demethylases are found either mutated, chromosomal translocated, or over-expressed when isolated from oncogenic cells, suggesting that they play an important regulatory role in the cell. Unique interactions have been identified that are being pursued to develop therapeutics. Currently, structural analyses are in progress to assist with targeting the interaction in an effort to disrupt in a specific manner.
Kimberly Buhman
College of Health and Human Sciences
Nutrition Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The long-term goal of the Buhman laboratory is to identify novel factors that regulate dietary fat sensing, metabolism or absorption that may be exploited for preventive and therapeutic interventions for obesity, diabetes, and heart disease. Research in the Buhman laboratory focuses on trafficking and metabolism of digestive products of dietary fat within the absorptive cells of the small intestine, enterocytes. Projects in the Buhman laboratory are currently addressing how diet, drugs and genetics affect chylomicron synthesis and secretion, cytoplasmic lipid droplets synthesis and metabolism, and fatty acid oxidation by enterocytes. Recent publications from the Buhman laboratory highlight important functions of diet, drugs, and genetics in regulation of dietary fat processing within enterocytes that results in effects related to metabolic diseases such as body weight, blood lipid concentrations, and hepatic steatosis.
Ryan Cabot
College of Agriculture
Animal Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The research conducted in our laboratory is focused on learning how the mammalian embryo directs its development from a single cell to a complex group of differentiated tissues and ultimately a fully formed adult organism. We are particularly interested in understanding how in vitro manipulation procedures affect development of the pig embryo and how these effects can be circumvented to improve embryo quality and embryo viability. It is well-established that many of the in vitro manipulations performed on mammalian embryos (e.g., in vitro production and culture of embryos) are correlated with increased rates of developmental failure and altered gene expression in surviving live-born animals. One technique in particular, cloning by nuclear transfer, has given scientists the ability to produce live-born domestic animals that harbor targeted genetic modifications.
The benefits from increasing the quality of embryos produced following in vitro manipulation will have a large impact on several scientific fields. First, it will allow us to increase the reproductive efficiency of agriculturally important species. Secondly, understanding how to better handle mammalian embryos in vitro will benefit the biomedical community as a resource to generate animal models for human diseases. While the scientific community has gained tremendous insight into the mechanisms of many human diseases through the use of transgenic and knock-out mice, much more sophisticated models, perhaps using animals that are more 'physiologically relevant', may be found in genetically modified livestock species, like the pig.
Current projects in the lab are aimed at examining the how specific epigenetic modifications are mediated in the early embryo (e.g., histone methylation) and the mechanisms by which specific chromatin-interacting factors access the nucleus during development.
Ignacio Camarillo
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Our lab integrates aspects of physiology, cell biology, and molecular biology to elucidate the mechanisms by which prolactin (PRL) and growth hormone (GH) regulate locally produced hormones, receptors and growth factors. The main goal of this research is to better understand the complex interactions between the mammary epithelia and stroma. These studies are critical to our understanding of breast cancer, given that circulating levels of GH and PRL can significantly influence mammary tumorigenesis. The study of normal mammary development will provide more specific roles for these hormones and can identify novel potential targets for cancer therapies.
Several hormones, including PRL and GH, are necessary for normal mammary gland development. Two primary components of the mammary gland are epithelial cells, which differentiate to produce milk, and stromal cells consisting primarily of adipocytes. The majority of studies examining PRL and GH action during mammary development have centered on their effects in epithelial cells. Recently, there has been increasing evidence indicating the mammary stroma is a rich source of lipids and growth factors that are critical for epithelial growth. The regulation of these stromal factors by PRL or GH is therefore an important question in mammalian physiology.
Gaurav Chopra
College of Science
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The overarching theme of our research is to develop and verify multiscale chemical models of cellular systems for therapeutic discovery by integrating sequence, structure, function, interaction, and systems-based methodologies. Our lab is a hybrid computational and wet-lab to identify drugs by taking into account all possible interactions between biomolecules, namely, interactome based drug discovery. We will focus on designing disease-specific compounds interacting with multiple proteomes and biomolecular interfaces (protein/protein and protein/nucleic-acid interfaces) and identifying compounds that change the fate and proliferation of cell types in vivo by developing structural/chemical signatures of individual cells. Specifically, we will start by repurposing human approved compounds and designing new compounds to perturb the immune system to identify therapeutics for cancer and autoimmune diseases. Developing computational chemistry/biology tools and using physical chemistry principles fuel the research work that we do. The experimental validations of the computational predictions will be done in our laboratory, together with existing and new collaborators. Our lab will make use of high performance computing to generate predictions, use high-throughput robotic set-up for compound screening on cell assays, use molecular biology techniques & sequencing (RNA-seq, ChIP-seq, ATAC-seq etc.), flow cytometry instrumentation as needed to select and test computational and in vitro validated predictions in mice.
Chittaranjan Das
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- X Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Cancer and bacterial infection by ESKAPE pathogens, Cancers caused by HPV infection
Molecular/Cellular Target(s)
UCHL1, UCHL3, UCHL5, bacterial DUBs and ligases, HPV E6 protein
Research Interest and Expertise
Drug development targeting UCH family of DUBs, viral and bacterial E3 ligases and DUBs Expertise: Biochemistry of human and pathogenic ubiquitinating and deubiquitinating enzymes. X-ray crystallography and structure-based drug design
Emily Dykhuizen
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Recent cancer genome sequencing studies have uncovered frequent mutations in genes encoding subunits of nuclear protein complexes involved in chromatin remodeling and epigenetic regulation. We are interested in using a combination of chemical and biochemical techniques to uncover the role of chromatin structure in tumor suppression. Uncovering the mechanisms of these complexes will reveal potential therapeutic avenues for cancers that currently have few therapeutic options, such as renal clear cell carcinoma and ovarian clear cell carcinoma.
Marxa Figueiredo
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Prostate cancer bone metastasis, Inflammatory Arthritis, cartilage repair in osteoarthritis
Molecular/Cellular Target(s)
Interleukin-27, Pigment Epithelium Derived Factor, Laminin Receptor 1
Research Interest and Expertise
Our laboratory aims to understand the interactions between the skeletal and immune systems with the goal to develop novel therapeutic applications. We focus on integrating biological mechanisms with development of strategies that can leverage the immune system to simultaneously promote restoration of bone and alter immune responses to control inflammation or cell viability. Our therapeutic modalities build on multifunctional osteo-immune cytokines, which can be targeted to bone or inflammatory cells in order to exert regenerative effects.
Jennifer Freeman
College of Health and Human Sciences
Health Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Neurodegenerative diseases with a specific current focus on Alzheimer’s disease
- Cancer with a specific current focus on melanoma and neuroendocrine cancers
- Reproductive function
Research Interest and Expertise
The Freeman laboratory is an environmental molecular toxicology laboratory with current research efforts focused on investigating the adverse health effects of exposure to environmental stressors on human and environmental health using the zebrafish model system. The zebrafish is a prominent model system in a variety of biological disciplines and has become one of the preferred vertebrate models in biomedical research.
Similarities between the zebrafish and human genome permits investigations into the molecular pathways found to play a role in the mechanisms of toxicity in the zebrafish and translation to humans. Ongoing research projects in the Freeman laboratory are defining the underlying genetic and epigenetic mechanisms of toxicity of environmental stressors with current focus on pesticides, metals, radiation, and other legacy and emerging contaminants. These projects are identifying genetic biomarkers and molecular pathways of the immediate adverse impacts of a developmental exposure, the lasting impacts of this developmental exposure throughout the lifespan, and the analysis of subsequent generations linking genetic, epigenetic, and phenotypic assessments. These studies are investigating a developmental origin of adult disease pathogenesis with a specific focus on cancer, reproductive function, and neurodegenerative disorders.
The Freeman laboratory has expertise in the application of the zebrafish model system and with genomic and targeted genetic and epigenetic technologies including array comparative genomic hybridization (CGH) to detect copy number variants and aberrations; transcriptomics including gene expression microarrays and sequencing to identify genetic biomarkers (i.e., gene targets) and molecular pathway alterations; and epigenetic analysis specifically with a focus on microRNA deregulation. All equipment and analysis platforms needed for microarray experiments are available in the Freeman laboratory.
Mark Hall
College of Agriculture
Biochemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The majority of our work is conducted in the budding yeast Saccharomyces cerevisiae. Budding yeast are easy to work with and manipulate genetically, making them an attractive model organism for studying conserved and fundamental biological processes, such as cell division. We apply biochemistry, cell biology, molecular biology, and genetics methods to our research projects, providing a diverse training experience for students. In addition, we use mass spectrometry in a variety of ways, particularly for the discovery, quantification, and characterization of protein-protein interactions and protein post-translational modifications.
Tony Hazbun
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Infectious diseases such as Candidiasis
- Prostate cancer
- Parkinson’s disease
- Prions
- Aging
Molecular/Cellular Target(s)
- Hsp90 chaperone
- Kinetochore proteins, Aurora kinase, Mitotic kinases
- Hsp31/DJ-1 chaperone
- Prions
Research Interest and Expertise
The Hazbun lab uses yeast as a functional genomics and systems biology tool to probe biological pathways involved in mitosis and protein homeostasis. The facility of yeast genetics and genomewide resources allows us to probe many different biological pathways involved in disease including Parkinsons Disease, Cancer and infectious disease.
A major biological focus of the lab is centered on the protein-protein interactions that occur in the assembly and function the kinetochore, an important macromolecular complex that is at the hub of chromosome segregation process. Aurora kinases are an important enzyme that phosphorylates many kinetochore proteins and regulates kinetochore function. They are overexpressed in many cancers and have been pursued as a therapeutic target although there has been limited success partly because of the limited approach taken by many pharmaceutical companies. We are investigating how the Aurora kinase and other mitotic kinases control protein-protein interactions at the kinetochore. Understanding this overall process and delineating how these interactions are controlled will allow us to develop a more targeted and designed approach to inhibit cellular proliferation.
An additional focus in the lab is the targeting of Hsp90, a chaperone protein for which tumor cells are preferentially dependent. Chaperones are proteins that assist in folding and increasing the activity of other protein substrates. We are identifying small molecule modulators of this chaperone and investigating their novel mechanisms of binding. Although several inhibitors exist for Hsp90, we have identified a novel inhibitor with a new mode of binding that results in unique biological response. We have recently published on another chaperone, Hsp31, which is the yeast homolog of DJ-1, a human protein implicated in Parkinson’s Disease. We have delineated and probed the multiple functions of this protein, which include chaperone activity and enzyme activities. We have also shown that Hsp31 can have a role in modulating prion status in yeast providing valuable insight into how Hsp31 intervenes when a protein is misfolded. Further work will focus on finding small molecules that can bind to Hsp31 and modulate its function.
A final project in the lab is to use yeast genomics to identify small molecule targets. We have implemented the haploinsufficiency chemogenomic profiling method in the lab to identify the targets of small molecules that have unclear or poorly defined mechanisms of action. We are currently focusing on antifungal compounds but the method can be used for small molecules involved in cancer or neurodegenerative disease depending on the potential target.
Catherine Hill
College of Agriculture
Entomology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Genomics of Arthropod Vectors of Human Disease: Our research program is focused on the genomics of arthropod vectors of human disease such as malaria, West Nile virus and Lyme disease. The overall objective of this research is the development of novel strategies to control arthropod disease vectors.
Molecular/Cellular Target(s)
Mosquito G Protein-coupled Receptors: Mosquito transmitted diseases such as malaria and dengue cause significant morbidity and mortality worldwide. Insecticide and drug resistance problems and lack of effective vaccines necessitate the development of novel approaches for mosquito and mosquito-borne disease control. G protein-coupled receptors (GPCRs) are highly desirable molecular targets due to their function in many fundamental biological processes such as chemo- and photoreception, development, neuro-physiology and stress response. We use bioinformatic, molecular and comparative genomics approaches to identify and characterize GPCRs in two major mosquito vectors of disease, the malaria mosquito Anopheles gambiae and the yellow fever mosquito, Aedes aegypti.
Research Interest and Expertise
Genomics of Ixodid Ticks: Ticks (subphylum Chelicerata, class Arachnida) transmit a diverse array of infectious agents and are second only to mosquitoes as vectors of human pathogens. Current knowledge of ixodid tick biology is limited and the genetic basis of phenotypes such as host location, vector competence and insecticide resistance is poorly understood. We are currently leading an international effort funded by the National Institutes of Health to sequence the first tick genome, namely the Lyme disease tick, Ixodes scapularis. In the USA, I. scapularis transmits the causative agents of Lyme disease, babesiosis and human granulocytic anaplasmosis. The Ixodes Genome Project (IGP), represents an unparalleled resource for studying tick biology and tick-host-pathogen relationships, and identifying novel targets for tick and tick-borne disease control. We are currently undertaking genomic and cytogenetic studies in the Ixodidae to understand tick chromosome biology and genome architecture and to facilitate genome assembly.
Gregory Hockerman
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Voltage-gated calcium channels are key players in a large array of physiological processes including contraction of cardiac, vascular and skeletal muscle, release of neurotransmitters from nerve terminals, gene expression, and hormone secretion. The long-range goal of our studies is to contribute to the development of drugs that can modulate voltage-gated calcium channels in a tissue and type selective manner to treat cardiovascular disease and type II diabetes.
Harm Hogenesch
Comparative Pathobiology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Dr. HogenEsch is a board-certified veterinary pathologist with 20 years of experience in immunology and pathology. His research focuses on vaccine development and the immunopathology of chronic inflammation. Dr. HogenEsch is an expert on aluminum-containing adjuvants, the only adjuvants allowed for use in human vaccines in the US. The HogenEsch lab investigates mechanisms by which aluminum-containing adjuvants enhance the immune response and develops methods to optimize the formulation of aluminum-adjuvanted vaccines. The research on chronic inflammation focuses on the role of the protein SHARPIN in inflammation. Dr. HogenEsch discovered the cpdm mouse mutant which is caused by a mutation in the Sharpin gene. The mutant mice develop a severe chronic eosinophilic dermatitis, systemic inflammation and defects in the development of lymphoid organs and in the Th1 immune response.
Chang-Deng Hu
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Prostate cancer
Molecular/Cellular Target(s)
AP1, CREB, PRMT5 and other PRMTs
Research Interest and Expertise
Regulation of gene expression at the transcriptional and epigenetic level is a key process to determine how cells respond to intracellular and extracellular signals. Because of this, deregulation of transcription factors and epigenetic regulators is often implicated in many human diseases such as cancer. We use molecular, cellular, biochemical, genetic, "Omics" and imaging approaches to identifying novel and unique molecular interactions at the transcriptional and epigenetic level that regulate the growth of cancer cells, determine the response of cancer cells to therapy, and confer the resistance to treatment. The ultimate goal is to develop novel therapeutics to treat cancer.
Rong Huang
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Andy Hudmon
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Hyun-Young Jeong
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Qing Jiang
College of Health and Human Sciences
Nutrition Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Chronic inflammation constitutes one of the major etiologies of degenerative diseases including cancer. My laboratory is interested in studying the molecular mechanism of inflammation-associated diseases, and exploring prevention and therapy of these diseases, using nutrition factors including natural forms of vitamin E as well as combinations of vitamin E forms and other antioxidants.
Wen Jiang
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Andrea Kasinski
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Lung and breast cancer
Molecular/Cellular Target(s)
- Non-coding RNAs
- Kras
- p53
- LIN28
- MYC
- MET
- miR-34
- let-7
Research Interest and Expertise
MicroRNAs (miRNAs) are small non-coding RNAs that posttranscriptionally regulate the expression of protein-coding genes. The discovery of miRNAs has resulted in a paradigm shift in our knowledge about gene control and therapeutic intervention. Through their binding to their target genes, these “master regulators” induce subtle alterations in gene expression that can culminate in major phenotypic changes. This is based on the notion that miRNAs are pleiotropic, referring to the fact that miRNAs can bind to and affect multiple targets. Although the expression of an individual miRNA target may only change marginally, the combined effect of suppressing several targets at the same time results in a phenotypic transformation. This is most clearly illustrated in the context of cancer where miRNA dysregulation contributes to many types of cancer. In some instances the combination of multiple subtle changes causes the tumor cells to become addicted to a single miRNA. MiR-21 and miR-155 are two oncogenic miRNAs (oncomiRs) that have shown this type of addictive pattern in vivo. Similarly loss of key tumor suppressive miRNAs, through epigenetic silencing, genomic loss, and reduced upstream signaling and processing, has been correlated with disease state. Based on this knowledge we have two major goals: i) to identify noncoding RNAs that drive tumorigenesis, specifically miRNAs, and ii) to utilize this knowledge to target miRNAs and their biogenesis pathways for cancer therapeutic.
Kee Hong Kim
Department Of Food Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Ann Kirchmaier
College of Agriculture
Biochemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Diseases associated with misregulated epigenetic processes
Molecular/Cellular Target(s)
Chromatin assembly factors, histone de/acetylases, histone de/methylases, histone variants, DNA methyltransferases, Tet oxygenases
Research Interest and Expertise
The research in my lab focuses on understanding how cells regulate epigenetic processes. Our research examines the role of the cell cycle and DNA replication in assembly or maintenance of chromatin structures and the effects of these structures on DNA replication. We are interested in how the cell restricts heterochromatin to specific genomic loci, why heterochromatin formation is regulated by the cell cycle, how transcription of genes is prevented in silenced regions, and whether epigenetic processes are influenced by or influence events including DNA damage and the initiation of DNA replication. We are intrigued by how epigenetic states are maintained throughout the cell cycle and are duplicated and inherited each time the chromosome itself is replicated and the cell divides. We investigate how environmental factors perturb epigenetic processes and can contribute to inappropriate gene expression, developmental defects, tumorogenesis and other catastrophic disorders.
Our research explores the interface between epigenetic processes, histone modifications, chromatin assembly, DNA replication and the cell cycle, Our laboratory combines molecular biology, biochemical and quantitative microscopy-based approaches with mammalian cell culture and the power of yeast genetics to understand the impact of genetic and external factors on epigenetic gene regulation.
Jeff Ko
College of Veterinary Medicine
Veterinary Clinical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
We specialize in anesthesia and pain management, obesity/metabolic syndrome animal models, cardiovascular- pulmonary dysfunctions, and spinal cord-CNS animal models, including:
- animal models of anesthesia and pain management
- inhalant and injectable anesthetics
- pain management techniques and drug delivery
- pain assessment and brain images with functional MRI
- anesthesia equipment
- anesthetic monitoring of cardiorespiratory and brain functions
Shihuan Kuang
College of Agriculture
Animal Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Obesity, Type 2 Diabetes, Muscular Dystrophy, Rhabdomyosarcoma, Liposarcoma
Molecular/Cellular Target(s)
Notch signaling pathway, PTEN, Lkb1/Stk11, mTOR
Research Interest and Expertise
Muscle stem cell biology and muscle regeneration: A balance between self-renewal and differentiation is crucial for stem cell maintenance and tissue homeostasis. However, mechanisms governing stem cell fate are poorly understood. One goal of our research is to address this question using muscle satellite cells as a model system. Several recent studies have revealed an important role of asymmetric division in satellite cell self-renewal. We are particularly interested in the role of Notch signaling in the cell fate decision of muscle satellite cells.
Skeletal muscles have a remarkable regenerative capacity due to myogenic differentiation of satellite cells. Deregulation and dysfunction of muscle stem cells lead to regenerative failure in aged muscle and a number of muscular dystrophy diseases. One focus of my lab is to explore the signaling mechanisms that regulate satellite cells and explore how such mechanisms are employed in muscle regeneration.
Adipose tissue plasticity and obesity: Adipose tissue contains white, beige (also called brite) and brown adipocytes. White adipocytes store lipids and excessive accumulation of lipids is associated with obesity. Beige and brown adipocytes can break down and utilize lipids to generate heat, and are associated with leaner body mass. We are particularly interested in the lineage origin of the three types of adipocytes and their plasticity (interconversion). To this end, my lab has discovered a novel role of Notch signaling in regulating adipocyte plasticity. Interestingly, aberrant activation of Notch signaling induces tumorgenic transformation of adipocytes, resulting in development of liposarcoma. Understanding the molecular mechanisms that regulate adipose tissue plasticity is key to the development of therapeutic approached to combat the rising epidemics of obesity and its associated metabolic syndromes.
Muscle-fat crosstalk: We have recently shown that muscle interstitial adipocytes are required for efficient regeneration of injured muscles. Meanwhile, we found that muscle-specific cytokines (myokines) can regulate the plasticity (for example conversion of white to beige adipcytes) and gene expression of adipose tissues. We use a variety of animal models to understand the key signaling pathways that regulate skeletal muscle and adipose tissue health. Understanding the molecular basis of muscle-fat interaction will ultimately leads to strategies to improve the regenerative capacity of skeletal muscles and prevent/treat obesity and diabetes.
Richard Kuhn
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Sophie Lelievre
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Research in the laboratory focuses on understanding the mechanisms by which the organization of components of the cell nucleus directs the expression and stability of the genome, and how tissue architecture influences nuclear organization. Our approach makes use of three-dimensional cultures of nonmalignant and malignant breast epithelial cells that recapitulate the formation of normal tissue structures (mammary acini) and tumor nodules, respectively. Using this system, nuclear structural proteins, including the nuclear mitotic apparatus protein NuMA, have been demonstrated to differentially relocate upon differentiation and tumorigenesis and their specific subcellular distribution has been shown to direct gene expression and cell behavior (e.g., invasive potential, proliferation, apoptosis). We have shown that NuMA participates in chromatin organization related to transcription control and DNA repair. Our current focus is to identify the binding partners of NuMA during differentiation and tumorigenesis and decipher the nuclear mechanisms by which NuMA controls higher order chromatin structures. We have unraveled sequences in NuMA that are potential regulators of its function and ligands involved in epigenetic mechanisms and response to environmental stress. This information will be used to further decipher the mechanisms by which nuclear structure controls cell phenotypes. A separate focus is to decipher the proteomic and genomic determinants of apical polarity, an important element of tissue architecture that is altered very early during breast cancer development. These determinants will be used as detection markers for the classification of breast cancers and as potential therapeutic targets. The effect of apical polarity on gene expression control is also being investigated. In addition, nanotechnology approaches, based on the use DNA tweezers, are being developed to control the expression of specific genes. These studies should yield strategies to fight against differentiation and proliferation disorders like cancers. Another area of research is the development of breast cancer prevention strategies by combining epigenomics research with the study of environmental risk and protective factors for breast tissue homeostasis. This aspect of the research is highly interdisciplinary and includes all aspects of public health necessary to develop prevention programs. Finally, collaborative endeavors with engineers are focused on the design of organ-on-a-chip models with readouts at the cell nucleus level for target discoveries and screening of drugs and materials for prevention and treatment of cancers.
Yuk Fai Leung
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Retinal degeneration
Molecular/Cellular Target(s)
Retinas, photoreceptors, retinal ganglion cells
Research Interest and Expertise
Retinal degeneration is a group of inherited eye diseases including retinitis pigmentosa and age-related macular degeneration that impair our vision. They are incurable, even though much has been learned about the molecular basis of these diseases. To expedite discovery of new drugs for these diseases, we study zebrafish retinal-degeneration models.
We focus on two research directions:
- Disease-causing gene network for retinal degeneration
- Drug discovery for retinal degeneration.
Please visit our lab website for further information.
Andrea Liceaga
Department Of Food Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Seung-Oe Lim
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Xing Liu
Biochemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Different types of cancers Obesity Atrial fibrillation
Molecular/Cellular Target(s)
Cullin-RING ubiquitin E3 ligases Cullin associated NEDD8 dissociated protein 1 and 2 (CAND1 and CAND2)
Research Interest and Expertise
Our research goal is to understand the regulatory mechanisms of protein degradation and what role they play in human health and disease. In eukaryotic cells, the majority of protein degradation is carried out by the ubiquitin-proteasome system (UPS). Proteins selected for degradation by the UPS are firstly tagged with the small protein ubiquitin, and the ubiquitin tag is subsequently recognized by the proteasome, leading to degradation of the ubiquitin-tagged protein. To tag proteins with ubiquitin, it requires the ubiquitin ligases to bring the target protein and the ubiquitin together, which enables the transfer of the ubiquitin to the target protein. The biggest family of ubiquitin ligases are known as “cullin–RING ligases (CRLs)”. CRLs play major regulatory roles in cells, through their ability of specifying the half-lives of many key regulatory proteins that control a broad spectrum of biological events. We are using a variety of approaches including biochemistry, biophysics, proteomics, and molecular genetics, to study members of the CRL family to understand how they work, what they do, and how their activities can be manipulated to benefit human health. Our research systems include cultured human cells and model plants.
Zhao-Qing Luo
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Angeline Lyon
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac hypertrophy, arrhythmias, heart failure
Molecular/Cellular Target(s)
Phospholipase C (PLC) and subfamilies
Research Interest and Expertise
We use a combination of X-ray crystallography and electron microscopy to gain structural insights into PLC regulation and activation. Structure-based hypotheses are validated through functional assays, and ultimately cell-based and whole animal studies.
Sandro Matosevic
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Seema Mattoo
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- X Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Sulma Mohammed
College of Veterinary Medicine
Comparative Pathobiology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Dr. Mohammed’s research interest is to develop a model to study breast cancer progression in women and discern strategies for prevention. Due to routine breast mammography, detection of noninvasive mammary intraepithelial lesions (IELs), such as normotypic hyperplasia, atypical hyperplasia, and duct carcinoma in situ, is increasingly frequent. These lesions are believed to signal increased risk of developing invasive breast carcinoma in women. Although chemotherapy to reverse these lesions or to prevent their progression is a promising new strategy, an animal model with spontaneous pre-cancerous mammary intraepithelial lesions is needed to evaluate the safety and efficacy of candidate compounds. In a DoD-funded project, Dr. Mohammed studies the dog as an animal model with spontaneous mammary lesions that are phenotypically and genetically similar to human intraepithelial lesions. The advantages of studying the dog as a model over the rodent model include spontaneous development of DCIS and invasive cancer (all subtypes including triple-negative tumors), an intact immune system, hormonal responsiveness, and response to human chemotherapies. Dr. Mohammed’s in collaboration with her colleagues in Department of Comparative Pathobiology have shown that spontaneous canine mammary premalignant lesions such as atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS) are similar to those of the human breast in term of developing spontaneously before mammary tumors, histologic diversity, and immunohistochemical profile of ER-α, PR, and HER-2 (these findings, Antuofermo et al., 2007; were featured on the cover page of AACR Journal of Cancer Epidemiology, Biomarkers and Prevention where the article was published accompanied by an editorial by Dr. Elaine Ostrander, (Chief, Cancer Genetics Branch, National Human Genome Research Institute, NIH, Bethesda, Maryland) and were spread by various news agencies. In addition, her lab showed that clustered micro-calcifications and other radiographic lesions, corresponding to BI-RAD criteria for human breast cancer screening, can be detected in the canine mammary glands. This work is important, as it will allow non-invasive evaluation of drug efficacy in prevention clinical trials. Furthermore, Dr. Mohammed lab has conducted genome-wide transcription and methylation studies of canine mammary lesions along the continuum of cancer progression in the same gland (with progressing and non-progressing DCIS) and identified 21 genes with differential methylation and altered expression including immune-related genes (NKG7, CCL5, IFGGD3 (IRGM), and IFGGB2). The ultimate goal of this work, using this canine model, is to determine the mechanisms mediating the progression of DCIS to invasive cancer.
Valerie O'Brien
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- X Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Gastric cancer, most cases of which are due to stomach infection with the bacterium Helicobacter pylori.
Molecular/Cellular Target(s)
Gastric and intestinal mucins; epidermal growth factor receptor signaling; KRAS; type 1 immunity.
Research Interest and Expertise
The O’Brien Lab uses microbiology, immunology, and cancer biology approaches to investigate how and why chronic bacterial infection causes gastric cancer, and to reveal new drug and biomarker targets.
Brian Overholser
College of Pharmacy
Department Of Pharmacy Practice
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
My research program is focused on the clinical pharmacology of cardiovascular drugs. The overall goal is to enhance the understanding of pathophysiological mechanisms and pharmacological factors influencing the response variability to cardiovascular active agents.
Chiwook Park
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Proteins are dynamic molecules. Even under native conditions, they do not adopt a single static conformation. Rather, they access many different conformations in their native state ensemble. This native state ensemble includes small fluctuations around the native conformation, partially unfolded forms, and even globally unfolded forms. The distribution of these conformations and the kinetic barriers between the conformational states define the conformational energy landscapes of proteins. My research interest is investigating conformational energy landscapes of proteins and deciphering the relationship between the energetics of proteins and their biochemical functions, such as catalysis, signal transduction, and ligand binding. We use proteolysis as a major tool to probe protein structures and dynamics as well as conventional spectroscopic methods. We also use proteomics extensively for investigating energy landscapes of proteins on a system level.
Elizabeth Parkinson
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Peristera Paschou
Biological Sciences
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Neurodevelopmental Disorders, Neurogenerative Disorders, Neuromuscular Disorders
Molecular/Cellular Target(s)
Genomic analysis, Neuroimaging analysis
Research Interest and Expertise
Dr Paschou works at the intersection of Data Science and genomics research. She studies human genetic variation around the world aiming to understand the cause of neurological and neuropsychiatric disorders and identify predictive biomarkers. She is an expert in genomewide association studies (GWAS) aiming to elucidate the genetic basis of complex disease and studies population genetic structure around the world. She has a long-standing interest in Tourette Syndrome (TS) and related neurodevelopmental disorders of childhood onset and leads multiple international consortia that are pioneering investigations in this field. She currently coordinates a mega-GWAS for TS and is Chair of the ENIGMA-Tourette Syndrome Working Group, bringing together genetics and neuroimaging datasets from multiple collaborating sites with a goal to understand brain structure and function in TS. She also works on understanding the link between neurodevelopment and neurodegeneration, focusing on the identification of predictive biomarkers for Alzheimer’s Disease and Related Dementias.
Elsje Pienaar
Biomedical Engineering
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Carol Post
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Molecular/Cellular Target(s)
- Tyrosine kinases
- Flavivirus capsid proteins
- synuclein
Research Interest and Expertise
Research interests of the Post group include the regulation and functional aspects of protein-protein interactions, enzymatic catalysis and activation, and protein structure. Multidimensional spin magnetic resonance methods are used to determine three-dimensional structures and internal dynamics of protein complexes. Computational methods are used to understand the structural basis for protein stability, protein-ligand binding energetics, enzymatic regulation and activity, and the mechanism of action of antiviral compounds.
Timothy Ratliff
College of Veterinary Medicine
Comparative Pathobiology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Prostate Cancer, Bladder Cancer, autoimmune disease
Molecular/Cellular Target(s)
Immune regulatory cells, prostate cancer cell, prostate stem cells
Research Interest and Expertise
Our laboratory focuses on understanding immune regulation and the development of alternative approaches to treating urologic cancers, primarily bladder and prostate cancers, through the modulation of anti-cancer immunity. Current studies focus on prostate inflammation, its immune regulation and its impact on prostate stem cells, gene expression in prostate tissue, cholesterol metabolism in prostate cancer and impact of inflammation on prostate cancer development. The intent is to develop a better understanding of the inflammatory factors contributing to cancer development and to use the information gained to develop novel approaches to treating prostate cancer through modulation of the immune response.
Genetically modified mouse models are used to probe inflammation, immune regulation, the development of autoimmunity and anticancer effector mechanisms. Regulatory cells termed myeloid-derived suppressor cells have been linked to regulation of prostate inflammation and are observed early in genetically modified mouse spontaneous prostate tumor models. Determining how to control myeloid-derived suppressor cell function is a focus that is anticipated to provide a new approach to augmenting antitumor immunity. Likewise, understanding the impact of inflammation on prostate stem cells is anticipated to advance knowledge about the mechanisms of prostate growth and cancer development.
Christopher Rice
Comparative Pathobiology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Several human and animal diseases caused by various pathogenic free-living amoeba. Acanthamoeba Keratitis (AK) – Acanthamoeba species. Granulomatous Amoebic Encephalitis (GAE) – Acanthamoeba species. Cutaneous skin lesions – Acanthamoeba species. Disseminated Acanthamoeba Infection – Acanthamoeba species.
Primary Amoebic Meningoencephalitis (PAM) – Naegleria fowleri.
Balamuthia Amoebic Encephalitis (BAE) or GAE – Balamuthia mandrillaris.
Cutaneous skin lesions – Balamuthia mandrillaris.
Disseminated Balamuthia Infection – Balamuthia mandrillaris.
Molecular/Cellular Target(s)
Most of our therapeutic targets have been suggested through phenotypic whole cell screening against each of these three pathogenic free-living amoebae ((FLA); Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris). This chemical inference approach identifies compounds which are active against the amoeba and then suggests a specific protein target. Please see https://www.ssgcid.org/ for an up-to-date list of requested targets for structural determination and their progress by our collaborators.
Research Interest and Expertise
My primary focus for the last decade has been identifying essential amino acid biochemical pathways through target and phenotypic based drug discovery on these amoebae. I have developed high-throughput screening methods to assess hundreds-to-thousands of compounds for drug repurposing or as chemical starting points to optimise into potential prophylactic or curative therapeutics in the future. My lab is interested in drug discovery and development for orphan diseases, host-pathogen interactions, understanding the diversity of species and variable pathobiology they may cause, using pathobiological in vivo models to assess disease and develop novel treatments for these devastating diseases.
Daniel Smith
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
The Center for Paralysis Research and Department of Industrial and Physical Pharmacy focus on neurological trauma, endeavoring to discover and develop drugs for the treatment of spinal cord injuries.
Robert Stahelin
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Andy Tao
College of Agriculture
Computer Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The Tao research group focuses on the development and applications of biological mass spectrometry for functional proteomics. Examining changes in proteins of interest and their modifications within cells under different physiological conditions will offer insights into understanding cellular and molecular mechanisms that cannot currently be obtained through traditional biological studies that usually focus on the detailed analysis of individual biomolecules. Functional proteomics thus holds significant promise for the discovery of diagnostic or prognostic protein markers, for the detection of new therapeutic targets, and as a powerful tool to further our understanding of basic biological processes and mechanisms. The realization of these expectations relies on the development of robust and highly sensitive and specific methods to identify and quantify important proteins and their specific modifications.
Isabelle Vanhaezebrouck
Veterinary Clinical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Yang Yang
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Synthesis/Optimization
Synthesis/Optimization

Ryan Altman
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Abram Axelrod
Chemistry
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Arun Bhunia
College of Agriculture/College of Veterinary Medicine
Department Of Food Science
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Pathogen and Toxin Detection:
- Biosensor technologies including laser light scattering, mammalian cell-based, and fiber optic sensors for rapid and high throughput screening of live pathogens and toxins in food
- Development of biorecognition including antibodies, receptors, ligands, and microbiological growth media
Host-Pathogen Interaction and Control Strategies Using Probiotics:
- Understanding the molecular and cellular mechanism of Listeria monocytogenes colonization and translocation through epithelial barrier during intestinal phase of infection
- Prevention and control using bioengineered probiotic and antimicrobial peptide loaded biocompatible nano-carrier
Jean Chmielewski
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Diseases resulting from pathogenic bacteria, including Mycobacterium tuberculosis, MRSA, VRSA, Salmonella, Shigella, Listeria, Brucella, Acinetobacter baumannii, and others
- Bacteria biofilms
- HIV reservoirs
- Drug-resistant malaria
Molecular/Cellular Target(s)
- Intracellular pathogenic bacteria with an emphasis on subcellular localizations within mammalian cells
- HIV reservoirs in the brain
- Drug resistant P. falciparum with an emphasis on the chloroquine-resistance transporter (PfCRT)
Research Interest and Expertise
- We have expertise in the design and synthesis of novel non-membrane lytic antibacterial peptides that penetrate mammalian cells and their reversible conjugates with other antimicrobial agents.
- We have developed potent Trojan horse inhibitors of multidrug resistance transporters present at the blood-brain-barrier that revert to therapeutic agents within mammalian cells. This strategy can be used to improve the uptake of therapies (antiviral and anti-cancer) into the brain for HIV reservoir and tumor eradication.
- We have designed novel inhibitors of PfCRT within P. falciparum that effectively reverses drug resistance in cell culture with anti-malaria activity and show efficacy in a mouse model.
Gaurav Chopra
College of Science
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The overarching theme of our research is to develop and verify multiscale chemical models of cellular systems for therapeutic discovery by integrating sequence, structure, function, interaction, and systems-based methodologies. Our lab is a hybrid computational and wet-lab to identify drugs by taking into account all possible interactions between biomolecules, namely, interactome based drug discovery. We will focus on designing disease-specific compounds interacting with multiple proteomes and biomolecular interfaces (protein/protein and protein/nucleic-acid interfaces) and identifying compounds that change the fate and proliferation of cell types in vivo by developing structural/chemical signatures of individual cells. Specifically, we will start by repurposing human approved compounds and designing new compounds to perturb the immune system to identify therapeutics for cancer and autoimmune diseases. Developing computational chemistry/biology tools and using physical chemistry principles fuel the research work that we do. The experimental validations of the computational predictions will be done in our laboratory, together with existing and new collaborators. Our lab will make use of high performance computing to generate predictions, use high-throughput robotic set-up for compound screening on cell assays, use molecular biology techniques & sequencing (RNA-seq, ChIP-seq, ATAC-seq etc.), flow cytometry instrumentation as needed to select and test computational and in vitro validated predictions in mice.
Mark Cushman
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Inhibitors and modulators of a wide variety of other targets are also being designed and synthesized, including topoisomerase II, tyrosyl-DNA phosphodiesterases 1 and 2, and Janus kinases 1, 2, and 3.
Daniel Flaherty
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Gram-negative infectious disease
- Staphylococcus aureus (MRSA) infection
- Breast cancer
- B-cell lymphoma
Molecular/Cellular Target(s)
- Ribonuclease E (Gram-negative pathogenic target)
- Ribonuclease P protein (S. aureus target)
- Ubiquitin C-terminal Hydrolase L1 (metastatic breast cancer and B-cell lymphoma target)
Research Interest and Expertise
Our laboratory employs a blend of traditional ligand-based medicinal chemistry with structure-based design techniques in early to intermediate stage drug discovery. We are primarily interested in novel therapeutic targets in the areas of infectious disease and cancer. We utilize our expertise to pursue quality modulators worthy of advancing as leads compounds. Our laboratory applies a tri-lateral approach to drug discovery incorporating fragment-based, traditional high-throughput screen based, and virtual HTS based techniques. We are heavily involved in screening and hit selection stages as well as design and synthesis of analogs. Furthermore, we provide a fragment-based screening platform in our lab that is available for investigators who would like to pursue a fragment-based approach against their targets and are in need of medicinal chemistry support.
Jessica Fortin
Basic Medical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Alzheimer’s Disease, Parkinson’s Disease, Type 2 Diabetes, Systemic Amyloidosis
Molecular/Cellular Target(s)
Prone-to-aggregate proteins: Alpha-synuclein Aβ1-40, Aβ1-42 Islet amyloid polypeptide (or amylin) Hyperphosphorylated tau (p-tau) Serum amyloid A1 (SAA1)
Research Interest and Expertise
My research lab focuses on the design and preparation of small molecules to inhibit the oligomer and fibril formation generated by prone-to-aggregate proteins.
R Edwin Garcia
College of Engineering
Materials Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Our group is interested in the development, integration and application of discrete and continuous analytical and numerical models to predict the processing-properties relationships and the mechanical reliability of pharmaceutical tablets. Of great interest is also the development of models and theories to design the dissolution and precipitation kinetics of pharmaceutical materials.
Arun Ghosh
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
My research group is involved in multidisciplinary research projects in the areas of synthetic organic, bioorganic and medicinal chemistry. Of particular interest, we are investigating:
- Synthesis and biological studies of Bioactive Natural Products
- Design and synthesis of Molecular Probes for Bioactive Peptides and Proteins
- Structure-based Design of Enzyme Inhibitors for Alzheimer's Disease and AIDS
- Development of Asymmetric methodologies (Catalytic and Stoichiometric)
- Multicomponent Reactions (MCR) for Highly Functionalized Products
Catherine Hill
College of Agriculture
Entomology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Genomics of Arthropod Vectors of Human Disease: Our research program is focused on the genomics of arthropod vectors of human disease such as malaria, West Nile virus and Lyme disease. The overall objective of this research is the development of novel strategies to control arthropod disease vectors.
Molecular/Cellular Target(s)
Mosquito G Protein-coupled Receptors: Mosquito transmitted diseases such as malaria and dengue cause significant morbidity and mortality worldwide. Insecticide and drug resistance problems and lack of effective vaccines necessitate the development of novel approaches for mosquito and mosquito-borne disease control. G protein-coupled receptors (GPCRs) are highly desirable molecular targets due to their function in many fundamental biological processes such as chemo- and photoreception, development, neuro-physiology and stress response. We use bioinformatic, molecular and comparative genomics approaches to identify and characterize GPCRs in two major mosquito vectors of disease, the malaria mosquito Anopheles gambiae and the yellow fever mosquito, Aedes aegypti.
Research Interest and Expertise
Genomics of Ixodid Ticks: Ticks (subphylum Chelicerata, class Arachnida) transmit a diverse array of infectious agents and are second only to mosquitoes as vectors of human pathogens. Current knowledge of ixodid tick biology is limited and the genetic basis of phenotypes such as host location, vector competence and insecticide resistance is poorly understood. We are currently leading an international effort funded by the National Institutes of Health to sequence the first tick genome, namely the Lyme disease tick, Ixodes scapularis. In the USA, I. scapularis transmits the causative agents of Lyme disease, babesiosis and human granulocytic anaplasmosis. The Ixodes Genome Project (IGP), represents an unparalleled resource for studying tick biology and tick-host-pathogen relationships, and identifying novel targets for tick and tick-borne disease control. We are currently undertaking genomic and cytogenetic studies in the Ixodidae to understand tick chromosome biology and genome architecture and to facilitate genome assembly.
Daniel Hogan
Veterinary Clinical Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac, vascular, thrombosis, valvular
Molecular/Cellular Target(s)
TGF-B and valvulopathy
Research Interest and Expertise
Our research focuses on cardiovascular therapeutics, including heart failure and antithrombotics. We have expertise in veterinary clinical trials, pre-clinical animal trials and animal modeling.
Rong Huang
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Qing Jiang
College of Health and Human Sciences
Nutrition Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Chronic inflammation constitutes one of the major etiologies of degenerative diseases including cancer. My laboratory is interested in studying the molecular mechanism of inflammation-associated diseases, and exploring prevention and therapy of these diseases, using nutrition factors including natural forms of vitamin E as well as combinations of vitamin E forms and other antioxidants.
Casey Krusemark
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Cancer
Molecular/Cellular Target(s)
- Protein Kinases (broadly)
- chromodomains
Research Interest and Expertise
Our work centers on the use of DNA-encoding approaches for discovery and development of biologically active small molecules. In one area, we utilize DNA-programmed combinatorial chemistry to construct novel chemical libraries of DNA-encoded small molecules. We are using these libraries to develop peptidomimetic inhibitors of protein-protein interactions. In a second area, we have developed a DNA-based assay approach for biochemical assays including several enzymatic assays and ligand binding assays. We work to apply these assays in proteomic activity profiling and in small molecule screening.
Our lab has extensive expertise in DNA-encoded chemical approaches and in design of DNA-compatible combinatorial chemical libraries. Additional expertise lies generally in the areas of bioconjugation chemistry, peptide/peptidomimetic synthesis, and DNA sequence analysis.
Mark Lipton
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Drug Resistant Bacterial infections
- Prostate cancer
- Prader-Willi Syndrome
Molecular/Cellular Target(s)
- Type II HMG CoA Reductase
- FabF
- Ghrelin O-Acyl Transferase
Research Interest and Expertise
Our research effort combines the disciplines of organic synthesis, bioorganic chemistry and molecular modeling. Current projects in the Lipton group fall into two areas:
Development of novel synthetic methodology: We are currently working on the development of new methods in the area of solid phase synthesis of larger and head-to-tail cyclized peptides.
Design and synthesis of biologically active molecules: Ongoing projects include the development of dual-action antimicrobials that target drug-resistant bacterial pathogens. These molecules are designed to inhibit two essential enzymes, type II HMG CoA Reductase and FabF. We also are making peptidic inhibitors of Ghrelin O-Acyl transferase (GOAT), an enxyme essential for the production of functional peptide hormone ghrelin. Ghrelin is associated with hunger as well as glucose homeostasis and it is believed that inhibition of GOAT may lead to new treatments for diabetes. A third project area is the production of a targeted chemotherapeutic agent designed to combat refractory prostate cancers through the conjugation of a cholesterol-depleting drug to DUPA, an agent that targets Prostate Specific Antigen.
Philip Low
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Cancers of the ovaries, prostate, cervix, kidneys, colon, lung, breast
- Autoimmune/Inflammatory diseases such as rheumatoid arthritis, atherosclerosis, pulmonary fibrosis, Crohn’s disease, osteoarthritis, psoriasis
- Influenza, malaria, HIV
- Obesity, diabetes
- Sickle cell disease
Molecular/Cellular Target(s)
Folate receptors (alpha, beta, and delta), carbonic anhydrase IX, CCK2R, prostate specific membrane antigen (PSMA), luteinizing hormone-releasing hormone (LHRH), bombesin receptor, aminopeptidase N, fibroblast activation protein, neuraminidase, red blood cell kinases, band 3
Research Interest and Expertise
To date, we have developed targeted therapeutic and/or imaging agents for a variety of cancers (e.g. ovarian, lung, kidney, endometrial, breast and prostate), several inflammatory diseases (rheumatoid arthritis, Crohn’s disease, osteoarthritis, organ transplant rejection, psoriasis, etc.), diabetes, atherosclerosis and a variety of infectious diseases (e.g. malaria, influenza virus, Staphylococcus, Pseudomonas, etc.). Eleven drugs stemming from research in my lab are currently undergoing human clinical trials (mainly at Endocyte, Inc., HuLow, and On Target Laboratories, three companies that I have founded).
Interests include: Imaging of malignant diseases; isolation and analysis of circulating tumor cells; fluorescence guided surgery using tumor-targeted fluorescent dyes; and personalized medicine, therapies for infectious diseases.
Elizabeth Parkinson
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Elsje Pienaar
Biomedical Engineering
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Padinjaremadhom Ramachandran
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Design and syntheses of complex molecular targets for the treatment of cancer, inflammation, and central nervous system disorders. Our current efforts focus on (1) the preparation of fluorinated analogs of the potent tubulin polymerizing agent, (-)-dictyostatin (2) the synthesis and biology of several fluorinated carbohydrates and nucleosides for the treatment of pancreatic cancer in collaboration with Indiana University School of Medicine. (3) synthesis of optically active GABA agonists/antagonists/potentiators and assay on various ion channels and GABA receptors for the treatment of epilepsy, neuropathic pain, and addiction through collaborations (Purdue Medicinal Chemistry Department and the Stark Neurosciences Research Institute).
Herman Sintim
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Acute Myeloid Leukemia
- Pancreatic Cancer
- Gram-(-) and (+) infections
Molecular/Cellular Target(s)
- Kinases
- G-quadruplexes
- Cyclic Dinucleotide Synthases
- Cyclic Dinucleotide Phosphodiesterases
Research Interest and Expertise
Research interests are new anticancer agents, the chemical biology of bacterial communication, virulence factors production and biofilm formation, the discovery of antibiotics with novel modes of action, the catalytic cycle of total syntheses of complex bioactive molecules, and the discovery of reaction methodologies and new DNA nanostructures and machines for bioanlyte detection.
Daniel Smith
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
The Center for Paralysis Research and Department of Industrial and Physical Pharmacy focus on neurological trauma, endeavoring to discover and develop drugs for the treatment of spinal cord injuries.
John Tesmer
Biological Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
David H. Thompson
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
We are developing novel synthetic chemical and biochemical tools to address fundamental problems in human health, with a special emphasis on cancer therapeutic agents, Niemann-Pick Type C therapeutics, delivery of nucleic acid anti-cancer agents, and accelerated protein structure determination. Development of efficient chemoseletive routes to these materials is a major focus of our research. We are also exploring the effects of particle shape, size, and environmentally responsive transformations (e.g., pH, enzyme, light, ultrasound) on therapeutic performance. Translation of these basic studies to animal models of disease (e.g., bladder, lung, pancreatic & breast tumors) is the near-term goal of our materials development efforts.
Darci Trader
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Parkinson’s, Huntington’s, HIV, Hepatitis C, Melanoma, Lymphoma
Molecular/Cellular Target(s)
In cancer we are targeting Rpn-6, an essential component of the 26S proteasome, and gankyrin, a chaperone folding protein essential for forming the proteasome. Our interest in protein-accumulation diseases, such as Parkinson’s and Huntington’s disease, is focused on developing new small molecule agonists that stimulate the 20S proteasome to degrade the protein fibrils associated with these diseases. For targeting HIV and hepatitis we are working towards discovering peptidomimetics that can prevent proteins associated with the previously mentioned viruses from inhibiting the immunoproteasome.
Research Interest and Expertise
Our main research interests lie in targeting the proteasome, through inhibition in cancer cells and stimulation in protein accumulation diseases. Additionally, we are also working towards developing small molecules that rescue the immunoproteasome from inhibition by virally-produced proteins and how to target this proteasome isoform as a prodrug release mechanism.
Christopher Uyeda
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Zhong-Yin Zhang
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Lung cancer, melanoma, glioblastoma, pancreatic cancer and other malignancies.
- Lupus, rheumatoid arthritis and other autoimmune disorders
- Diabetes and obesity
- Tuberculosis
Molecular/Cellular Target(s)
SHP2, PRL, PTP1B, Lyp, LMW-PTP, mPTPA and mPTPB
Research Interest and Expertise
Research in this laboratory spans the disciplines of chemistry and biology with an emphasis on the structure and function of protein tyrosine phosphatases (PTPs), roles of PTP in normal physiology and pathological conditions, and the design and synthesis of PTP inhibitors as chemical probes to interrogate PTP function and as novel therapeutics.
- Portfolio of PTP inhibitors for oncology, diabetes, autoimmune disorders and infectious diseases
- PTP-based drugs will be first-in-class
- Recognized world leader in PTP chemistry and biochemistry
- Unparalleled expertise in the PTP space - over 30 years of experience in the area
- PTP-dedicated lab with substantial medicinal chemistry, structure-based design, structural biology and molecular modeling, assay development, high throughput screening, cell biology and in vivo testing.
- Largest collection of PTPs and assays for selectivity profiling
Wei Zheng
College of Health and Human Sciences
Health Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
- Molecular mechanisms, biomarkers and chelation therapy of manganese (Mn)-induced Parkinsonian disorder.
- Molecular mechanisms by which lead (Pb) exposure alters brain transport and homeostasis of beta-amyloid, which contributes to the etiology of Alzheimer’s disease.
- Adult neurogenesis in metal-induced neurotoxicities and contributions of brain barrier systems in adult neurogenesis.
- Transport of substances (metals, polypeptides, and drug molecules) by the blood-brain barrier and blood-CSF barrier.
- Integrity of brain barriers in neurodegenerative diseases and obesity-associated neurobehavioral alterations.
Delivery/Formulations
Delivery/Formulations
Ryan Altman
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Xiaoping Bao
Chemical Engineering
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Jean Chmielewski
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Diseases resulting from pathogenic bacteria, including Mycobacterium tuberculosis, MRSA, VRSA, Salmonella, Shigella, Listeria, Brucella, Acinetobacter baumannii, and others
- Bacteria biofilms
- HIV reservoirs
- Drug-resistant malaria
Molecular/Cellular Target(s)
- Intracellular pathogenic bacteria with an emphasis on subcellular localizations within mammalian cells
- HIV reservoirs in the brain
- Drug resistant P. falciparum with an emphasis on the chloroquine-resistance transporter (PfCRT)
Research Interest and Expertise
- We have expertise in the design and synthesis of novel non-membrane lytic antibacterial peptides that penetrate mammalian cells and their reversible conjugates with other antimicrobial agents.
- We have developed potent Trojan horse inhibitors of multidrug resistance transporters present at the blood-brain-barrier that revert to therapeutic agents within mammalian cells. This strategy can be used to improve the uptake of therapies (antiviral and anti-cancer) into the brain for HIV reservoir and tumor eradication.
- We have designed novel inhibitors of PfCRT within P. falciparum that effectively reverses drug resistance in cell culture with anti-malaria activity and show efficacy in a mouse model.
Marxa Figueiredo
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Prostate cancer bone metastasis, Inflammatory Arthritis, cartilage repair in osteoarthritis
Molecular/Cellular Target(s)
Interleukin-27, Pigment Epithelium Derived Factor, Laminin Receptor 1
Research Interest and Expertise
Our laboratory aims to understand the interactions between the skeletal and immune systems with the goal to develop novel therapeutic applications. We focus on integrating biological mechanisms with development of strategies that can leverage the immune system to simultaneously promote restoration of bone and alter immune responses to control inflammation or cell viability. Our therapeutic modalities build on multifunctional osteo-immune cytokines, which can be targeted to bone or inflammatory cells in order to exert regenerative effects.
R Edwin Garcia
College of Engineering
Materials Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Our group is interested in the development, integration and application of discrete and continuous analytical and numerical models to predict the processing-properties relationships and the mechanical reliability of pharmaceutical tablets. Of great interest is also the development of models and theories to design the dissolution and precipitation kinetics of pharmaceutical materials.
Arun Ghosh
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
My research group is involved in multidisciplinary research projects in the areas of synthetic organic, bioorganic and medicinal chemistry. Of particular interest, we are investigating:
- Synthesis and biological studies of Bioactive Natural Products
- Design and synthesis of Molecular Probes for Bioactive Peptides and Proteins
- Structure-based Design of Enzyme Inhibitors for Alzheimer's Disease and AIDS
- Development of Asymmetric methodologies (Catalytic and Stoichiometric)
- Multicomponent Reactions (MCR) for Highly Functionalized Products
Catherine Hill
College of Agriculture
Entomology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Genomics of Arthropod Vectors of Human Disease: Our research program is focused on the genomics of arthropod vectors of human disease such as malaria, West Nile virus and Lyme disease. The overall objective of this research is the development of novel strategies to control arthropod disease vectors.
Molecular/Cellular Target(s)
Mosquito G Protein-coupled Receptors: Mosquito transmitted diseases such as malaria and dengue cause significant morbidity and mortality worldwide. Insecticide and drug resistance problems and lack of effective vaccines necessitate the development of novel approaches for mosquito and mosquito-borne disease control. G protein-coupled receptors (GPCRs) are highly desirable molecular targets due to their function in many fundamental biological processes such as chemo- and photoreception, development, neuro-physiology and stress response. We use bioinformatic, molecular and comparative genomics approaches to identify and characterize GPCRs in two major mosquito vectors of disease, the malaria mosquito Anopheles gambiae and the yellow fever mosquito, Aedes aegypti.
Research Interest and Expertise
Genomics of Ixodid Ticks: Ticks (subphylum Chelicerata, class Arachnida) transmit a diverse array of infectious agents and are second only to mosquitoes as vectors of human pathogens. Current knowledge of ixodid tick biology is limited and the genetic basis of phenotypes such as host location, vector competence and insecticide resistance is poorly understood. We are currently leading an international effort funded by the National Institutes of Health to sequence the first tick genome, namely the Lyme disease tick, Ixodes scapularis. In the USA, I. scapularis transmits the causative agents of Lyme disease, babesiosis and human granulocytic anaplasmosis. The Ixodes Genome Project (IGP), represents an unparalleled resource for studying tick biology and tick-host-pathogen relationships, and identifying novel targets for tick and tick-borne disease control. We are currently undertaking genomic and cytogenetic studies in the Ixodidae to understand tick chromosome biology and genome architecture and to facilitate genome assembly.
Daniel Hogan
Veterinary Clinical Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac, vascular, thrombosis, valvular
Molecular/Cellular Target(s)
TGF-B and valvulopathy
Research Interest and Expertise
Our research focuses on cardiovascular therapeutics, including heart failure and antithrombotics. We have expertise in veterinary clinical trials, pre-clinical animal trials and animal modeling.
Christine Hrycyna
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The overall goal of my research program is to understand the mechanisms and roles of important eukaryotic integral membrane proteins that are fundamental to human health and disease. My multidisciplinary work successfully integrates the tools of biochemistry, molecular biology, cell biology and biophysical chemistry to define how these membrane proteins recognize their substrates and how they operate at the molecular level. We also develop ways to use our mechanistic knowledge to create pharmacological agents to modulate the activities of these important proteins. Specifically, I focus on three major areas: 1) the membrane-associated enzymes involved in the posttranslational processing of ) the human ATP binding cassette (ABC) transporters ABCG2 and P-glycoprotein, and (3) drug discovery for inhibitors of human Icmt and for human ABC transporters at the blood-brain barrier.
Wen Jiang
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Andrea Kasinski
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Lung and breast cancer
Molecular/Cellular Target(s)
- Non-coding RNAs
- Kras
- p53
- LIN28
- MYC
- MET
- miR-34
- let-7
Research Interest and Expertise
MicroRNAs (miRNAs) are small non-coding RNAs that posttranscriptionally regulate the expression of protein-coding genes. The discovery of miRNAs has resulted in a paradigm shift in our knowledge about gene control and therapeutic intervention. Through their binding to their target genes, these “master regulators” induce subtle alterations in gene expression that can culminate in major phenotypic changes. This is based on the notion that miRNAs are pleiotropic, referring to the fact that miRNAs can bind to and affect multiple targets. Although the expression of an individual miRNA target may only change marginally, the combined effect of suppressing several targets at the same time results in a phenotypic transformation. This is most clearly illustrated in the context of cancer where miRNA dysregulation contributes to many types of cancer. In some instances the combination of multiple subtle changes causes the tumor cells to become addicted to a single miRNA. MiR-21 and miR-155 are two oncogenic miRNAs (oncomiRs) that have shown this type of addictive pattern in vivo. Similarly loss of key tumor suppressive miRNAs, through epigenetic silencing, genomic loss, and reduced upstream signaling and processing, has been correlated with disease state. Based on this knowledge we have two major goals: i) to identify noncoding RNAs that drive tumorigenesis, specifically miRNAs, and ii) to utilize this knowledge to target miRNAs and their biogenesis pathways for cancer therapeutic.
Gregory Knipp
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Drug transporters
Molecular/Cellular Target(s)
Early, Intermediate
Research Interest and Expertise
I currently serve as the Associate Director of the Dane O. Kildsig-Center for Pharmaceutical Processing Research and the Director of the Purdue Translational Pharmacology CTSI core. My specific interests lie in the preclinical and early translational development ADMET research for facilitating NCE selection and optimization. General research interests include:
- The molecular and functional characterization of drug transporters
- Determining the effect of xenobiotics on placental fatty acid homeostasis and fetal development.
- Investigating the effects of excipients on drug transport
- in vitro cell permeability studies for drug screening
- GI cell lines: NCI-N87 (gastric epithelium), Caco-2 (Small intestine), and HT-29 (Colon)
- Peripheral vasculature and Blood Brain Barrier: HUVECs, hCMEC/D3, and primary human cells
- Placental: HRP-1, Rcho-1, and BeWo.
- Other Cell Lines: COS-7, THP-1, HeLa
- In vivo PK work with the rodent Culex and large animal Culex-L porcine models.
- Utilization of the porcine model for testing the PK assessment of drug formulations.
- Preclinical formulation development
- Recently developed a novel 3D human blood brain barrier in vitro model for drug screening.
Shihuan Kuang
College of Agriculture
Animal Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Obesity, Type 2 Diabetes, Muscular Dystrophy, Rhabdomyosarcoma, Liposarcoma
Molecular/Cellular Target(s)
Notch signaling pathway, PTEN, Lkb1/Stk11, mTOR
Research Interest and Expertise
Muscle stem cell biology and muscle regeneration: A balance between self-renewal and differentiation is crucial for stem cell maintenance and tissue homeostasis. However, mechanisms governing stem cell fate are poorly understood. One goal of our research is to address this question using muscle satellite cells as a model system. Several recent studies have revealed an important role of asymmetric division in satellite cell self-renewal. We are particularly interested in the role of Notch signaling in the cell fate decision of muscle satellite cells.
Skeletal muscles have a remarkable regenerative capacity due to myogenic differentiation of satellite cells. Deregulation and dysfunction of muscle stem cells lead to regenerative failure in aged muscle and a number of muscular dystrophy diseases. One focus of my lab is to explore the signaling mechanisms that regulate satellite cells and explore how such mechanisms are employed in muscle regeneration.
Adipose tissue plasticity and obesity: Adipose tissue contains white, beige (also called brite) and brown adipocytes. White adipocytes store lipids and excessive accumulation of lipids is associated with obesity. Beige and brown adipocytes can break down and utilize lipids to generate heat, and are associated with leaner body mass. We are particularly interested in the lineage origin of the three types of adipocytes and their plasticity (interconversion). To this end, my lab has discovered a novel role of Notch signaling in regulating adipocyte plasticity. Interestingly, aberrant activation of Notch signaling induces tumorgenic transformation of adipocytes, resulting in development of liposarcoma. Understanding the molecular mechanisms that regulate adipose tissue plasticity is key to the development of therapeutic approached to combat the rising epidemics of obesity and its associated metabolic syndromes.
Muscle-fat crosstalk: We have recently shown that muscle interstitial adipocytes are required for efficient regeneration of injured muscles. Meanwhile, we found that muscle-specific cytokines (myokines) can regulate the plasticity (for example conversion of white to beige adipcytes) and gene expression of adipose tissues. We use a variety of animal models to understand the key signaling pathways that regulate skeletal muscle and adipose tissue health. Understanding the molecular basis of muscle-fat interaction will ultimately leads to strategies to improve the regenerative capacity of skeletal muscles and prevent/treat obesity and diabetes.
Philip Low
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Cancers of the ovaries, prostate, cervix, kidneys, colon, lung, breast
- Autoimmune/Inflammatory diseases such as rheumatoid arthritis, atherosclerosis, pulmonary fibrosis, Crohn’s disease, osteoarthritis, psoriasis
- Influenza, malaria, HIV
- Obesity, diabetes
- Sickle cell disease
Molecular/Cellular Target(s)
Folate receptors (alpha, beta, and delta), carbonic anhydrase IX, CCK2R, prostate specific membrane antigen (PSMA), luteinizing hormone-releasing hormone (LHRH), bombesin receptor, aminopeptidase N, fibroblast activation protein, neuraminidase, red blood cell kinases, band 3
Research Interest and Expertise
To date, we have developed targeted therapeutic and/or imaging agents for a variety of cancers (e.g. ovarian, lung, kidney, endometrial, breast and prostate), several inflammatory diseases (rheumatoid arthritis, Crohn’s disease, osteoarthritis, organ transplant rejection, psoriasis, etc.), diabetes, atherosclerosis and a variety of infectious diseases (e.g. malaria, influenza virus, Staphylococcus, Pseudomonas, etc.). Eleven drugs stemming from research in my lab are currently undergoing human clinical trials (mainly at Endocyte, Inc., HuLow, and On Target Laboratories, three companies that I have founded).
Interests include: Imaging of malignant diseases; isolation and analysis of circulating tumor cells; fluorescence guided surgery using tumor-targeted fluorescent dyes; and personalized medicine, therapies for infectious diseases.
Zhao-Qing Luo
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Sandro Matosevic
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Andrew Mesecar
College of Science
Purdue Center For Cancer Research
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The fundamental research interests of the Mesecar lab involve elucidating the molecular mechanisms and function of therapeutic enzymes and proteins. We wish to understand at the molecular level how enzymes and proteins recognize their substrates, catalyze their requisite chemical reactions, and trigger signal-transduction cascades. Our ultimate goal is to utilize this fundamental scientific knowledge to develop new therapeutics to treat cancer and infectious diseases.
To achieve these goals, we integrate a variety of state-of-the-art research tools and approaches including X-ray crystallography, enzyme chemistry and kinetics, molecular biology, bioinformatics, mass spectrometry, and computational chemistry to gain an understanding of the role of protein dynamics and conformational change in molecular recognition and catalysis. We then couple these technologies with high-throughput screening and structure-based design to develop compounds capable of modulating the activity of enzymes and receptors involved in cancer chemoprevention, cancer cell proliferation, cell longevity, and bacterial and viral pathogenesis.
Suresh Mittal
College of Veterinary Medicine
Comparative Pathobiology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
- Pandemic Influenza Vaccine
- Adenoviral Vectors
- Cancer Gene Therapy
Kinam Park
College of Pharmacy
Biomedical Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
His research has been focused on the use of various polymers and hydrogels for controlled drug delivery. His current research includes homogeneous nano/microparticles using nanofabrication, hydrotropic polymeric micelles, superporous hydrogels, fast melting tablet formulations, and drug-eluting stents. He has published more than 200 peer-reviewed papers, 70 book chapters, and presented 190 abstracts at national and international meetings. He has also made more than 200 invited lectures throughout the world. He co-authored and co-edited 7 books in the area of controlled drug delivery, and edited special journal issues in the area of protein- and cell-repellent surfaces and in the area of hydrogels. He has trained more than 80 Ph.D. graduate students, postdoctoral fellows and visiting scientists. He founded Akina, Inc. specializing in drug delivery technologies in 2001.
Elsje Pienaar
Biomedical Engineering
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Rodolfo Pinal
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Dr. Pinal’s research interests include: solution chemistry, solubility and solubilization techniques, mixtures, polymer-based composites as means of control of product performance (mechanical strength, dissolution, release rate and bioavailability) of bioactive compounds, antiplasticization, and molecular relaxation in amorphous organic materials.
Doraiswami Ramkrishna
College of Engineering
Chemical Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Professor Ramkrishna's research group is motivated by ideas in the application of mathematics to solving problems in chemical and biochemical reaction engineering, biotechnology and biomedical engineering. Their research ideas arise from linear (operator methods) and nonlinear analysis of ordinary and partial differential equations, stochastic processes, and population balance modeling involving integro-partial differential equations.
Christopher Rice
Comparative Pathobiology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Several human and animal diseases caused by various pathogenic free-living amoeba. Acanthamoeba Keratitis (AK) – Acanthamoeba species. Granulomatous Amoebic Encephalitis (GAE) – Acanthamoeba species. Cutaneous skin lesions – Acanthamoeba species. Disseminated Acanthamoeba Infection – Acanthamoeba species.
Primary Amoebic Meningoencephalitis (PAM) – Naegleria fowleri.
Balamuthia Amoebic Encephalitis (BAE) or GAE – Balamuthia mandrillaris.
Cutaneous skin lesions – Balamuthia mandrillaris.
Disseminated Balamuthia Infection – Balamuthia mandrillaris.
Molecular/Cellular Target(s)
Most of our therapeutic targets have been suggested through phenotypic whole cell screening against each of these three pathogenic free-living amoebae ((FLA); Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris). This chemical inference approach identifies compounds which are active against the amoeba and then suggests a specific protein target. Please see https://www.ssgcid.org/ for an up-to-date list of requested targets for structural determination and their progress by our collaborators.
Research Interest and Expertise
My primary focus for the last decade has been identifying essential amino acid biochemical pathways through target and phenotypic based drug discovery on these amoebae. I have developed high-throughput screening methods to assess hundreds-to-thousands of compounds for drug repurposing or as chemical starting points to optimise into potential prophylactic or curative therapeutics in the future. My lab is interested in drug discovery and development for orphan diseases, host-pathogen interactions, understanding the diversity of species and variable pathobiology they may cause, using pathobiological in vivo models to assess disease and develop novel treatments for these devastating diseases.
Jean-Christophe Rochet
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Parkinson’s disease
Molecular/Cellular Target(s)
Alpha-synuclein
Research Interest and Expertise
Research in the Rochet laboratory is aimed at understanding the role of protein aggregation in neurodegenerative disorders, with an emphasis on Parkinson's disease (PD). PD, an age-related neurodegenerative disorder that disrupts the lives of an estimated 5 million people worldwide, is manifested by classic motor symptoms including resting tremor, slowness of movement, rigidity, and postural instability. These symptoms result largely from a loss of dopaminergic neurons from the substantia nigra in the midbrain, and this neuronal loss is thought to involve oxidative stress and aggregation of the presynaptic protein α-synuclein (aSyn). Current therapies only temporarily relieve symptoms without slowing the underlying neurodegenerative disease. In addition, a large proportion of neurons have been destroyed by the time PD symptoms are detectable, and no therapies exist to reverse this damage. Accordingly, there is a critical need for neuroprotective strategies to help reduce the risk of PD.
Dr. Rochet’s lab has taken the approach of characterizing gene products involved in aging and neurodegenerative disorders (e.g. alpha-synuclein, DJ-1), with the aim of elucidating mechanisms of neuronal death and dysfunction. His group’s research involves an interdisciplinary approach with methods ranging from biochemical analyses of recombinant proteins to characterization of neurotoxic and neuroprotective mechanisms in cellular and animal models. Dr. Rochet’s studies in models that reproduce key aspects of PD pathobiology have yielded new insights into genetic and chemical suppressors of neurodegeneration.
Sandra Rossie
College of Agriculture
Biochemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Reversible phosphorylation is an important and common mechanism for regulating a wide variety of processes ranging from cellular excitation to gene expression. In contrast to our knowledge of protein kinases and their roles in these processes, we know far less about protein phosphatases and their regulation. Protein phosphatase 5 (PP5) is a recently described member of the largest Ser/Thr protein phosphatase family, with a unique N-terminal domain that inhibits PP5 activity and binds other proteins. Little is known about PP5's biological function, however this enzyme is implicated in controlling cell growth and in hormone signal transduction pathways. We are using biochemical and molecular approaches to define the role and regulation of PP5 in brain and other tissues. Projects are focused on the structural basis for controlling PP5 activity, identification of physiologic substrates and regulators for PP5, and examination of the regional and subcellular distribution of PP5. These studies will advance our understanding of PP5's function and the role that Ser/Thr protein phosphatases play in signal transduction pathways in brain and elsewhere.
Dennis Savaiano
College of Health and Human Sciences
Nutrition Science
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Our research group has studied numerous factors which influence lactose digestion and tolerance including lactose load, gastric and intestinal transit, the use of lactose digestive aids, colon fermentation of lactose and the consumption of fermented dairy foods and lactic acid bacteria. Major findings from these studies include: (1) The identification of a microbial lactase in yogurts that assists lactose digestion in the intestinal tract following the consumption of yogurt. (2) The characterization of the amount of lactose required to cause symptoms in lactose maldigesters, being 12g or more of lactose (one cup of milk). (3) The finding that lactose consumed with a meal is tolerated about 3 times better than lactose consumed in a fasted state. (4) Identifying the colonic flora as key in determining tolerance to lactose. The colonic flora readily adapts to lactose in the diet of maldigesters. Thus, maldigesters who routinely consume lactose have less symptoms due to more efficient metabolism of lactose by the colon microflora. (5) The identification of a population of digesters and maldigesters who believe that they are extremely intolerant to lactose, but who tolerate lactose quite well in double-blinded clinical trials. (6) The characterization of the ability of lactic acid bacteria including acidophilus and bifidus to improve lactose digestion in vivo in the gastro-intestinal system.
Garth Simpson
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Our research group is devoted to the theoretical development and experimental application of new instrumental methods taking advantage of unique nonlinear optical interactions. Recent interests include detection and analysis of crystals formed from chiral molecules, building on a long-standing interest in understanding the role of chirality and polarization-dependent effects in nonlinear optics.
Herman Sintim
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Acute Myeloid Leukemia
- Pancreatic Cancer
- Gram-(-) and (+) infections
Molecular/Cellular Target(s)
- Kinases
- G-quadruplexes
- Cyclic Dinucleotide Synthases
- Cyclic Dinucleotide Phosphodiesterases
Research Interest and Expertise
Research interests are new anticancer agents, the chemical biology of bacterial communication, virulence factors production and biofilm formation, the discovery of antibiotics with novel modes of action, the catalytic cycle of total syntheses of complex bioactive molecules, and the discovery of reaction methodologies and new DNA nanostructures and machines for bioanlyte detection.
Daniel Smith
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
The Center for Paralysis Research and Department of Industrial and Physical Pharmacy focus on neurological trauma, endeavoring to discover and develop drugs for the treatment of spinal cord injuries.
Lynne Taylor
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Our overall goal is to enhance drug delivery by optimizing and understanding the physicochemical properties of drugs and excipients. Of particular interest are amorphous solid dispersions which are used to improve the oral delivery of poorly water-soluble drugs. We are also interested in the stability of pharmaceutical salts and the impact of excipients on product performance. For these types of solids, it is extremely important to understand drug-excipient interactions as well as the impact of water on stability. We achieve an improved molecular level understanding of pharmaceutical materials and formulations through the use of high resolution analytical techniques. Some of the analytical techniques that we use are infrared, Raman, ultraviolet and fluorescence spectroscopy, X-ray powder diffraction and differential scanning calorimetry.
David H. Thompson
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
We are developing novel synthetic chemical and biochemical tools to address fundamental problems in human health, with a special emphasis on cancer therapeutic agents, Niemann-Pick Type C therapeutics, delivery of nucleic acid anti-cancer agents, and accelerated protein structure determination. Development of efficient chemoseletive routes to these materials is a major focus of our research. We are also exploring the effects of particle shape, size, and environmentally responsive transformations (e.g., pH, enzyme, light, ultrasound) on therapeutic performance. Translation of these basic studies to animal models of disease (e.g., bladder, lung, pancreatic & breast tumors) is the near-term goal of our materials development efforts.
Elizabeth Topp
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Alexander Wei
College of Science
Chemistry
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Cancer, ovarian and bladder (using orthotopic animal models)
- Bacterial infections
Molecular/Cellular Target(s)
- SKOV3 cells
- Tumor-associated macrophages
- Bacillus anthracis
- Chlamydia trachomatis
- Listeria monocytogenes
- Pseudomonas aeruginosa
- Salmonella enterica
- Staphylococcus aureus / MRSA
- Streptococcus pneumoniae
- Yersinia enterocolitica
- Albumin receptors
- Hemin receptors (Isd, etc.)
- Siderophore receptors (FoxA, FhuD2, FhuE)
Research Interest and Expertise
- Targeting ligands for pathogen detection and treatment, with particular interests in respiratory-tract and sexually transmitted infections.
- Targeted photodynamic therapy/inactivation (PDT / PDI) using photoactive hemin derivaties
- Identifying key serum proteins as mediators in nanoparticle tracking and cell uptake
- siRNA uptake and release in ovarian cancer cells
Michael Wendt
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Breast cancer
Molecular/Cellular Target(s)
EGFR, Her2, FGFR
Research Interest and Expertise
Research in the Wendt is focused on the role of epithelial-mesenchymal transition (EMT) in breast cancer metastasis. EMT is associated with resistance to several chemotherapeutic drugs and targeted molecular compounds. Recent studies by the Wendt has identified fibroblast growth factor receptor (FGFR) as major driver of drug resistance, particularly in the metastatic setting. Furthermore, cells that have undergone EMT become preferentially sensitive to inhibition of FGFR kinase activity. Work in the Wendt utilizes 3D cell culture and in vivo disease modeling in combination with an array of small molecule and biological approaches to optimize FGFR targeting for the treatment of metastatic and drug resistant breast cancer.
Danzhou Yang
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Molecular/Cellular Target(s)
DNA secondary structures and interactive proteins
Research Interest and Expertise
DNA-targeted anticancer drugs and structure-based rational drug design; Structures and functions of DNA secondary structures as cancer-specific molecular targets; DNA G-quadruplex secondary structures and their interactions with small molecule drugs and proteins; DNA-targeted anticancer drugs that inhibit transcription factors and topoisomerases. High-field NMR macromolecule structure determination.
Yuan Yao
Department Of Food Science
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Yoon Yeo
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Daoguo Zhou
College of Science
Biological Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Salmonellosis
- peptic ulcers
- stomach cancer
Molecular/Cellular Target(s)
- Type III secretion
- Type IV secretion
Research Interest and Expertise
The Zhou group focuses on the cell biology of infectious diseases, in particular human intestinal diseases caused by pathogenic Salmonella, E. coli and more recently Helicobacter pylori. These pathogens utilize the protein secretion/translocation system to inject bacterial “effector proteins” into host cells to exploit host cell functions to survive in the hostile environment and cause inflammatory responses. Using modern biochemical, cellular and microbiological approaches, they aim to understand the molecular and cellular mechanism of how these effectors function to enable the pathogens to circumvent the host immune system to cause diseases. They currently have projects studying the role(s) of actin dynamics in infections and how bacterial effectors exploit the host signaling pathways to induce inflammatory responses.
Qi Zhou
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
In Vivo, ADME, DMPK, TOX
In Vivo, ADME, DMPK, TOX

Kimberly Buhman
College of Health and Human Sciences
Nutrition Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The long-term goal of the Buhman laboratory is to identify novel factors that regulate dietary fat sensing, metabolism or absorption that may be exploited for preventive and therapeutic interventions for obesity, diabetes, and heart disease. Research in the Buhman laboratory focuses on trafficking and metabolism of digestive products of dietary fat within the absorptive cells of the small intestine, enterocytes. Projects in the Buhman laboratory are currently addressing how diet, drugs and genetics affect chylomicron synthesis and secretion, cytoplasmic lipid droplets synthesis and metabolism, and fatty acid oxidation by enterocytes. Recent publications from the Buhman laboratory highlight important functions of diet, drugs, and genetics in regulation of dietary fat processing within enterocytes that results in effects related to metabolic diseases such as body weight, blood lipid concentrations, and hepatic steatosis.
Michael Childress
College of Veterinary Medicine
Veterinary Clinical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Lymphomas
- Spontaneous canine cancer models
Research Interest and Expertise
My primary research interest is in canine and feline hematopoietic neoplasia, particularly canine lymphomas. I am a board-certified veterinary oncologist with extensive experience in the clinical management of canine and feline cancers.
Gaurav Chopra
College of Science
Chemistry
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The overarching theme of our research is to develop and verify multiscale chemical models of cellular systems for therapeutic discovery by integrating sequence, structure, function, interaction, and systems-based methodologies. Our lab is a hybrid computational and wet-lab to identify drugs by taking into account all possible interactions between biomolecules, namely, interactome based drug discovery. We will focus on designing disease-specific compounds interacting with multiple proteomes and biomolecular interfaces (protein/protein and protein/nucleic-acid interfaces) and identifying compounds that change the fate and proliferation of cell types in vivo by developing structural/chemical signatures of individual cells. Specifically, we will start by repurposing human approved compounds and designing new compounds to perturb the immune system to identify therapeutics for cancer and autoimmune diseases. Developing computational chemistry/biology tools and using physical chemistry principles fuel the research work that we do. The experimental validations of the computational predictions will be done in our laboratory, together with existing and new collaborators. Our lab will make use of high performance computing to generate predictions, use high-throughput robotic set-up for compound screening on cell assays, use molecular biology techniques & sequencing (RNA-seq, ChIP-seq, ATAC-seq etc.), flow cytometry instrumentation as needed to select and test computational and in vitro validated predictions in mice.
Marxa Figueiredo
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Prostate cancer bone metastasis, Inflammatory Arthritis, cartilage repair in osteoarthritis
Molecular/Cellular Target(s)
Interleukin-27, Pigment Epithelium Derived Factor, Laminin Receptor 1
Research Interest and Expertise
Our laboratory aims to understand the interactions between the skeletal and immune systems with the goal to develop novel therapeutic applications. We focus on integrating biological mechanisms with development of strategies that can leverage the immune system to simultaneously promote restoration of bone and alter immune responses to control inflammation or cell viability. Our therapeutic modalities build on multifunctional osteo-immune cytokines, which can be targeted to bone or inflammatory cells in order to exert regenerative effects.
Jessica Fortin
Basic Medical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Alzheimer’s Disease, Parkinson’s Disease, Type 2 Diabetes, Systemic Amyloidosis
Molecular/Cellular Target(s)
Prone-to-aggregate proteins: Alpha-synuclein Aβ1-40, Aβ1-42 Islet amyloid polypeptide (or amylin) Hyperphosphorylated tau (p-tau) Serum amyloid A1 (SAA1)
Research Interest and Expertise
My research lab focuses on the design and preparation of small molecules to inhibit the oligomer and fibril formation generated by prone-to-aggregate proteins.
Daniel Hogan
Veterinary Clinical Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac, vascular, thrombosis, valvular
Molecular/Cellular Target(s)
TGF-B and valvulopathy
Research Interest and Expertise
Our research focuses on cardiovascular therapeutics, including heart failure and antithrombotics. We have expertise in veterinary clinical trials, pre-clinical animal trials and animal modeling.
Andy Hudmon
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Andrea Kasinski
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Lung and breast cancer
Molecular/Cellular Target(s)
- Non-coding RNAs
- Kras
- p53
- LIN28
- MYC
- MET
- miR-34
- let-7
Research Interest and Expertise
MicroRNAs (miRNAs) are small non-coding RNAs that posttranscriptionally regulate the expression of protein-coding genes. The discovery of miRNAs has resulted in a paradigm shift in our knowledge about gene control and therapeutic intervention. Through their binding to their target genes, these “master regulators” induce subtle alterations in gene expression that can culminate in major phenotypic changes. This is based on the notion that miRNAs are pleiotropic, referring to the fact that miRNAs can bind to and affect multiple targets. Although the expression of an individual miRNA target may only change marginally, the combined effect of suppressing several targets at the same time results in a phenotypic transformation. This is most clearly illustrated in the context of cancer where miRNA dysregulation contributes to many types of cancer. In some instances the combination of multiple subtle changes causes the tumor cells to become addicted to a single miRNA. MiR-21 and miR-155 are two oncogenic miRNAs (oncomiRs) that have shown this type of addictive pattern in vivo. Similarly loss of key tumor suppressive miRNAs, through epigenetic silencing, genomic loss, and reduced upstream signaling and processing, has been correlated with disease state. Based on this knowledge we have two major goals: i) to identify noncoding RNAs that drive tumorigenesis, specifically miRNAs, and ii) to utilize this knowledge to target miRNAs and their biogenesis pathways for cancer therapeutic.
Kee Hong Kim
Department Of Food Science
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Deborah Knapp
College of Veterinary Medicine
Veterinary Clinical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
In our focus area of invasive urinary bladder cancer, we are defining heritable (through very strong dog breed- associated risk) and environmental risk factors. This will facilitate cancer prevention research in a highly relevant model in a very timely fashion. Because prevention studies in dogs can be performed in 1-3 years, dog studies can be used to select the most promising approach for the longer-term (15+ years) human studies. Our group is also studying cancer treatments including nanoparticles (in collaboration with Dr. James Leary), folate targeted therapy (in collaboration with Dr. Philip Low), demethylating agents (in collaboration with Dr. Noah Hahn, Indiana University School of Medicine) and with already established drugs (cyclooxygenase inhibitors, oral chemotherapies) being applied in a more effective dosing schedule.
Shihuan Kuang
College of Agriculture
Animal Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Obesity, Type 2 Diabetes, Muscular Dystrophy, Rhabdomyosarcoma, Liposarcoma
Molecular/Cellular Target(s)
Notch signaling pathway, PTEN, Lkb1/Stk11, mTOR
Research Interest and Expertise
Muscle stem cell biology and muscle regeneration: A balance between self-renewal and differentiation is crucial for stem cell maintenance and tissue homeostasis. However, mechanisms governing stem cell fate are poorly understood. One goal of our research is to address this question using muscle satellite cells as a model system. Several recent studies have revealed an important role of asymmetric division in satellite cell self-renewal. We are particularly interested in the role of Notch signaling in the cell fate decision of muscle satellite cells.
Skeletal muscles have a remarkable regenerative capacity due to myogenic differentiation of satellite cells. Deregulation and dysfunction of muscle stem cells lead to regenerative failure in aged muscle and a number of muscular dystrophy diseases. One focus of my lab is to explore the signaling mechanisms that regulate satellite cells and explore how such mechanisms are employed in muscle regeneration.
Adipose tissue plasticity and obesity: Adipose tissue contains white, beige (also called brite) and brown adipocytes. White adipocytes store lipids and excessive accumulation of lipids is associated with obesity. Beige and brown adipocytes can break down and utilize lipids to generate heat, and are associated with leaner body mass. We are particularly interested in the lineage origin of the three types of adipocytes and their plasticity (interconversion). To this end, my lab has discovered a novel role of Notch signaling in regulating adipocyte plasticity. Interestingly, aberrant activation of Notch signaling induces tumorgenic transformation of adipocytes, resulting in development of liposarcoma. Understanding the molecular mechanisms that regulate adipose tissue plasticity is key to the development of therapeutic approached to combat the rising epidemics of obesity and its associated metabolic syndromes.
Muscle-fat crosstalk: We have recently shown that muscle interstitial adipocytes are required for efficient regeneration of injured muscles. Meanwhile, we found that muscle-specific cytokines (myokines) can regulate the plasticity (for example conversion of white to beige adipcytes) and gene expression of adipose tissues. We use a variety of animal models to understand the key signaling pathways that regulate skeletal muscle and adipose tissue health. Understanding the molecular basis of muscle-fat interaction will ultimately leads to strategies to improve the regenerative capacity of skeletal muscles and prevent/treat obesity and diabetes.
Yuk Fai Leung
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Retinal degeneration
Molecular/Cellular Target(s)
Retinas, photoreceptors, retinal ganglion cells
Research Interest and Expertise
Retinal degeneration is a group of inherited eye diseases including retinitis pigmentosa and age-related macular degeneration that impair our vision. They are incurable, even though much has been learned about the molecular basis of these diseases. To expedite discovery of new drugs for these diseases, we study zebrafish retinal-degeneration models.
We focus on two research directions:
- Disease-causing gene network for retinal degeneration
- Drug discovery for retinal degeneration.
Please visit our lab website for further information.
Seung-Oe Lim
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Suresh Mittal
College of Veterinary Medicine
Comparative Pathobiology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
- Pandemic Influenza Vaccine
- Adenoviral Vectors
- Cancer Gene Therapy
Sulma Mohammed
College of Veterinary Medicine
Comparative Pathobiology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Dr. Mohammed’s research interest is to develop a model to study breast cancer progression in women and discern strategies for prevention. Due to routine breast mammography, detection of noninvasive mammary intraepithelial lesions (IELs), such as normotypic hyperplasia, atypical hyperplasia, and duct carcinoma in situ, is increasingly frequent. These lesions are believed to signal increased risk of developing invasive breast carcinoma in women. Although chemotherapy to reverse these lesions or to prevent their progression is a promising new strategy, an animal model with spontaneous pre-cancerous mammary intraepithelial lesions is needed to evaluate the safety and efficacy of candidate compounds. In a DoD-funded project, Dr. Mohammed studies the dog as an animal model with spontaneous mammary lesions that are phenotypically and genetically similar to human intraepithelial lesions. The advantages of studying the dog as a model over the rodent model include spontaneous development of DCIS and invasive cancer (all subtypes including triple-negative tumors), an intact immune system, hormonal responsiveness, and response to human chemotherapies. Dr. Mohammed’s in collaboration with her colleagues in Department of Comparative Pathobiology have shown that spontaneous canine mammary premalignant lesions such as atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS) are similar to those of the human breast in term of developing spontaneously before mammary tumors, histologic diversity, and immunohistochemical profile of ER-α, PR, and HER-2 (these findings, Antuofermo et al., 2007; were featured on the cover page of AACR Journal of Cancer Epidemiology, Biomarkers and Prevention where the article was published accompanied by an editorial by Dr. Elaine Ostrander, (Chief, Cancer Genetics Branch, National Human Genome Research Institute, NIH, Bethesda, Maryland) and were spread by various news agencies. In addition, her lab showed that clustered micro-calcifications and other radiographic lesions, corresponding to BI-RAD criteria for human breast cancer screening, can be detected in the canine mammary glands. This work is important, as it will allow non-invasive evaluation of drug efficacy in prevention clinical trials. Furthermore, Dr. Mohammed lab has conducted genome-wide transcription and methylation studies of canine mammary lesions along the continuum of cancer progression in the same gland (with progressing and non-progressing DCIS) and identified 21 genes with differential methylation and altered expression including immune-related genes (NKG7, CCL5, IFGGD3 (IRGM), and IFGGB2). The ultimate goal of this work, using this canine model, is to determine the mechanisms mediating the progression of DCIS to invasive cancer.
Valerie O'Brien
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- X Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Gastric cancer, most cases of which are due to stomach infection with the bacterium Helicobacter pylori.
Molecular/Cellular Target(s)
Gastric and intestinal mucins; epidermal growth factor receptor signaling; KRAS; type 1 immunity.
Research Interest and Expertise
The O’Brien Lab uses microbiology, immunology, and cancer biology approaches to investigate how and why chronic bacterial infection causes gastric cancer, and to reveal new drug and biomarker targets.
Christopher Rice
Comparative Pathobiology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Several human and animal diseases caused by various pathogenic free-living amoeba. Acanthamoeba Keratitis (AK) – Acanthamoeba species. Granulomatous Amoebic Encephalitis (GAE) – Acanthamoeba species. Cutaneous skin lesions – Acanthamoeba species. Disseminated Acanthamoeba Infection – Acanthamoeba species.
Primary Amoebic Meningoencephalitis (PAM) – Naegleria fowleri.
Balamuthia Amoebic Encephalitis (BAE) or GAE – Balamuthia mandrillaris.
Cutaneous skin lesions – Balamuthia mandrillaris.
Disseminated Balamuthia Infection – Balamuthia mandrillaris.
Molecular/Cellular Target(s)
Most of our therapeutic targets have been suggested through phenotypic whole cell screening against each of these three pathogenic free-living amoebae ((FLA); Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris). This chemical inference approach identifies compounds which are active against the amoeba and then suggests a specific protein target. Please see https://www.ssgcid.org/ for an up-to-date list of requested targets for structural determination and their progress by our collaborators.
Research Interest and Expertise
My primary focus for the last decade has been identifying essential amino acid biochemical pathways through target and phenotypic based drug discovery on these amoebae. I have developed high-throughput screening methods to assess hundreds-to-thousands of compounds for drug repurposing or as chemical starting points to optimise into potential prophylactic or curative therapeutics in the future. My lab is interested in drug discovery and development for orphan diseases, host-pathogen interactions, understanding the diversity of species and variable pathobiology they may cause, using pathobiological in vivo models to assess disease and develop novel treatments for these devastating diseases.
Jean-Christophe Rochet
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Parkinson’s disease
Molecular/Cellular Target(s)
Alpha-synuclein
Research Interest and Expertise
Research in the Rochet laboratory is aimed at understanding the role of protein aggregation in neurodegenerative disorders, with an emphasis on Parkinson's disease (PD). PD, an age-related neurodegenerative disorder that disrupts the lives of an estimated 5 million people worldwide, is manifested by classic motor symptoms including resting tremor, slowness of movement, rigidity, and postural instability. These symptoms result largely from a loss of dopaminergic neurons from the substantia nigra in the midbrain, and this neuronal loss is thought to involve oxidative stress and aggregation of the presynaptic protein α-synuclein (aSyn). Current therapies only temporarily relieve symptoms without slowing the underlying neurodegenerative disease. In addition, a large proportion of neurons have been destroyed by the time PD symptoms are detectable, and no therapies exist to reverse this damage. Accordingly, there is a critical need for neuroprotective strategies to help reduce the risk of PD.
Dr. Rochet’s lab has taken the approach of characterizing gene products involved in aging and neurodegenerative disorders (e.g. alpha-synuclein, DJ-1), with the aim of elucidating mechanisms of neuronal death and dysfunction. His group’s research involves an interdisciplinary approach with methods ranging from biochemical analyses of recombinant proteins to characterization of neurotoxic and neuroprotective mechanisms in cellular and animal models. Dr. Rochet’s studies in models that reproduce key aspects of PD pathobiology have yielded new insights into genetic and chemical suppressors of neurodegeneration.
Val Watts
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Neuropathic/inflammatory Pain
- Parkinson’s disease
- Drug Addiction
- Excitotoxicity
- Aging
- Infectious disease
Molecular/Cellular Target(s)
- D1 and D2 Dopamine Receptors
- Adenylyl Cyclases (1-9)
- Invertebrate Receptors
Research Interest and Expertise
The research in our laboratory is designed to use a multi-disciplinary approach, combining molecular biology, pharmacology, and biochemistry, to study the pharmacology and signaling mechanisms of G protein-coupled receptors (GPCRs). The fact that GPCRs are the target of approximately 50% of today’s clinically used drugs emphasizes further the relevance of these studies. Much of the work in our lab has focused on members of the dopamine, serotonin, and adenosine receptor families. Studies have examined the pharmacology of these receptors including the characterization of novel ligands that activate these receptors as well as examining the receptors’ ability to modulate the activity of their primary effector, the enzyme adenylyl cyclase. Recent efforts have focused on exploring the “druggability” of membrane adenylyl cyclase isoforms for human conditions. Additional efforts include drug discovery in the area of insecticides with Dr. Catherine Hill (Entomology). Together we have identified more than 100 different GPCRs in the genomes of the Aedes aegypti mosquito vector of Zika and dengue fever, the Anopheles gambiae mosquito vector of malaria, the Culex quinquefasciatus mosquito vector of filariasis and encephalitis, and the Ixodes scapularis tick vector of Lyme disease as potential drug targets. In summary, the goals of Dr. Watts’ research are to provide important information describing the biochemical changes associated with both short- and long-term activation of GPCRs and to identify novel novel receptor ligands and signaling pathways.
Michael Wendt
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Breast cancer
Molecular/Cellular Target(s)
EGFR, Her2, FGFR
Research Interest and Expertise
Research in the Wendt is focused on the role of epithelial-mesenchymal transition (EMT) in breast cancer metastasis. EMT is associated with resistance to several chemotherapeutic drugs and targeted molecular compounds. Recent studies by the Wendt has identified fibroblast growth factor receptor (FGFR) as major driver of drug resistance, particularly in the metastatic setting. Furthermore, cells that have undergone EMT become preferentially sensitive to inhibition of FGFR kinase activity. Work in the Wendt utilizes 3D cell culture and in vivo disease modeling in combination with an array of small molecule and biological approaches to optimize FGFR targeting for the treatment of metastatic and drug resistant breast cancer.
Yang Yang
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Ryan Altman
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Jessica Fortin
Basic Medical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Alzheimer’s Disease, Parkinson’s Disease, Type 2 Diabetes, Systemic Amyloidosis
Molecular/Cellular Target(s)
Prone-to-aggregate proteins: Alpha-synuclein Aβ1-40, Aβ1-42 Islet amyloid polypeptide (or amylin) Hyperphosphorylated tau (p-tau) Serum amyloid A1 (SAA1)
Research Interest and Expertise
My research lab focuses on the design and preparation of small molecules to inhibit the oligomer and fibril formation generated by prone-to-aggregate proteins.
David Foster
College of Pharmacy
Department Of Pharmacy Practice
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
My research interests are focused on the study of alterations in drug and nutrient disposition and drug effects in critically ill patients. Current research includes evaluation of changes in intestinal permeability to xenobiotics in critical illness. Specifically, this research involves the investigation of alterations in drug and nutrient absorption by passive and active transport mechanisms, and the molecular mediators underlying these changes in burn injury and sepsis. A related area of research is the use of natural anti-inflammatory compounds to attenuate inflammation-related changes in intestinal function. Other interests include the study of the contribution of active transport processes to variability in drug disposition in a number of patient populations. Dr. Foster's clinical interests are focused the provision of pharmacotherapy to critically-ill patients, with an emphasis on burn and trauma patients.
Hyun-Young Jeong
Industrial And Physical Pharmacy
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Gregory Knipp
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Drug transporters
Molecular/Cellular Target(s)
Early, Intermediate
Research Interest and Expertise
I currently serve as the Associate Director of the Dane O. Kildsig-Center for Pharmaceutical Processing Research and the Director of the Purdue Translational Pharmacology CTSI core. My specific interests lie in the preclinical and early translational development ADMET research for facilitating NCE selection and optimization. General research interests include:
- The molecular and functional characterization of drug transporters
- Determining the effect of xenobiotics on placental fatty acid homeostasis and fetal development.
- Investigating the effects of excipients on drug transport
- in vitro cell permeability studies for drug screening
- GI cell lines: NCI-N87 (gastric epithelium), Caco-2 (Small intestine), and HT-29 (Colon)
- Peripheral vasculature and Blood Brain Barrier: HUVECs, hCMEC/D3, and primary human cells
- Placental: HRP-1, Rcho-1, and BeWo.
- Other Cell Lines: COS-7, THP-1, HeLa
- In vivo PK work with the rodent Culex and large animal Culex-L porcine models.
- Utilization of the porcine model for testing the PK assessment of drug formulations.
- Preclinical formulation development
- Recently developed a novel 3D human blood brain barrier in vitro model for drug screening.
Shuang Liu
College of Health and Human Sciences
Health Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
More than 70 million Americans live with cardiovascular diseases. Accurate diagnosis is highly desirable so that appropriate therapeutic regimens can be given before irreversible damage occurs in the patients with known or suspected coronary artery disease (CAD). Myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) is an integral component in routine clinical evaluation of CAD patients. In spite of recent development of stress echocardiography and coronary CT angiography, SPECT MPI remains the mainstay for noninvasive diagnosis of CAD.
Molecular/Cellular Target(s)
Cardiolipin as the Molecular Target for Diagnosis of Heart Diseases. Heart is one of the organs rich with mitochondria. The mitochondrial density is as high as 40% of the cellular volume in myocytes. It is not surprising that mitochondrion has been a target for development of myocardial perfusion radiotracers that tend to localize inside the mitochondrial matrix. In contrast, CL is embedded in the inner mitochondrial membrane and constitutes up to as high as ~20% of its total lipid content. The fact that CL alterations underlie the myocardial dysfunction makes CL a useful and multifunctional biomarker for cardiovascular diseases (particularly HF), and provides the conceptual basis to develop molecular imaging probes that can be used to measure early CL changes noninvasively in the HF patients and those with diabetes.
Research Interest and Expertise
I worked at DuPont Medical Imaging Division (new Lantheus Medical Imaging Inc.) for nine years, and have research interests include receptor-based target radiopharmaceuticals, new bifunctional chelators, development of new techniques for radiolabeling of small biomolecules, formulation development, design/synthesis/evaluation of metal complexes as MRI contrast agents for cardiac perfusion imaging, and coordination chemistry of radiopharmaceuticals. There have been tremendous research efforts from his research group in the development of novel radiotracers for early tumor detection and diagnosis of cardiovascular diseases. These efforts rely on identification and the use of small biomolecules as “vehicles” to carry a diagnostic radionuclide to the tumor cells. Imaging with radiolabeled small biomolecules allows us to monitor the tumor biological changes at the molecular level. Over the last 10 years, Dr. Liu has become the leader in radiolabeled cyclic RGD peptides as integrin αvβ3-specific SPECT and PET radiotracers for imaging the integrin expression αvβ3 in rapidly growing and metastatic tumors. Dr. Liu is the author or co-author over 160 scientific publications, and has been granted 30 US patents and PCT applications. Dr. Liu’s contributions also have significant impacts on inorganic chemistry, radiochemistry, radiopharmaceutical development, bioconjugates chemistry, molecular imaging, and nuclear medicine. His research has been supported by grants from the National Institute of Health, Department of Energy, American Heart Association, and industry.
Christopher Rice
Comparative Pathobiology
Category of Research
- X Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Specific Disease(s)
Several human and animal diseases caused by various pathogenic free-living amoeba. Acanthamoeba Keratitis (AK) – Acanthamoeba species. Granulomatous Amoebic Encephalitis (GAE) – Acanthamoeba species. Cutaneous skin lesions – Acanthamoeba species. Disseminated Acanthamoeba Infection – Acanthamoeba species.
Primary Amoebic Meningoencephalitis (PAM) – Naegleria fowleri.
Balamuthia Amoebic Encephalitis (BAE) or GAE – Balamuthia mandrillaris.
Cutaneous skin lesions – Balamuthia mandrillaris.
Disseminated Balamuthia Infection – Balamuthia mandrillaris.
Molecular/Cellular Target(s)
Most of our therapeutic targets have been suggested through phenotypic whole cell screening against each of these three pathogenic free-living amoebae ((FLA); Naegleria fowleri, Acanthamoeba species, and Balamuthia mandrillaris). This chemical inference approach identifies compounds which are active against the amoeba and then suggests a specific protein target. Please see https://www.ssgcid.org/ for an up-to-date list of requested targets for structural determination and their progress by our collaborators.
Research Interest and Expertise
My primary focus for the last decade has been identifying essential amino acid biochemical pathways through target and phenotypic based drug discovery on these amoebae. I have developed high-throughput screening methods to assess hundreds-to-thousands of compounds for drug repurposing or as chemical starting points to optimise into potential prophylactic or curative therapeutics in the future. My lab is interested in drug discovery and development for orphan diseases, host-pathogen interactions, understanding the diversity of species and variable pathobiology they may cause, using pathobiological in vivo models to assess disease and develop novel treatments for these devastating diseases.
Val Watts
College of Pharmacy
Medicinal Chem/Molecular Pharmacology
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- X ADME/DMPK/Tox
- Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
- Neuropathic/inflammatory Pain
- Parkinson’s disease
- Drug Addiction
- Excitotoxicity
- Aging
- Infectious disease
Molecular/Cellular Target(s)
- D1 and D2 Dopamine Receptors
- Adenylyl Cyclases (1-9)
- Invertebrate Receptors
Research Interest and Expertise
The research in our laboratory is designed to use a multi-disciplinary approach, combining molecular biology, pharmacology, and biochemistry, to study the pharmacology and signaling mechanisms of G protein-coupled receptors (GPCRs). The fact that GPCRs are the target of approximately 50% of today’s clinically used drugs emphasizes further the relevance of these studies. Much of the work in our lab has focused on members of the dopamine, serotonin, and adenosine receptor families. Studies have examined the pharmacology of these receptors including the characterization of novel ligands that activate these receptors as well as examining the receptors’ ability to modulate the activity of their primary effector, the enzyme adenylyl cyclase. Recent efforts have focused on exploring the “druggability” of membrane adenylyl cyclase isoforms for human conditions. Additional efforts include drug discovery in the area of insecticides with Dr. Catherine Hill (Entomology). Together we have identified more than 100 different GPCRs in the genomes of the Aedes aegypti mosquito vector of Zika and dengue fever, the Anopheles gambiae mosquito vector of malaria, the Culex quinquefasciatus mosquito vector of filariasis and encephalitis, and the Ixodes scapularis tick vector of Lyme disease as potential drug targets. In summary, the goals of Dr. Watts’ research are to provide important information describing the biochemical changes associated with both short- and long-term activation of GPCRs and to identify novel novel receptor ligands and signaling pathways.
Other Areas
of Research
Other Areas
of Research

Keith Bowman
Materials Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
- Mechanical processing and properties of active ingredients and excipients
- Thermal and mechanical effects on crystalline solids
- X-ray diffraction
Stephen Byrn
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The Center for Paralysis Research and Department of Industrial and Physical Pharmacy focus on neurological trauma, endeavoring to discover and develop drugs for the treatment of spinal cord injuries.
Ryan Cabot
College of Agriculture
Animal Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
The research conducted in our laboratory is focused on learning how the mammalian embryo directs its development from a single cell to a complex group of differentiated tissues and ultimately a fully formed adult organism. We are particularly interested in understanding how in vitro manipulation procedures affect development of the pig embryo and how these effects can be circumvented to improve embryo quality and embryo viability. It is well-established that many of the in vitro manipulations performed on mammalian embryos (e.g., in vitro production and culture of embryos) are correlated with increased rates of developmental failure and altered gene expression in surviving live-born animals. One technique in particular, cloning by nuclear transfer, has given scientists the ability to produce live-born domestic animals that harbor targeted genetic modifications.
The benefits from increasing the quality of embryos produced following in vitro manipulation will have a large impact on several scientific fields. First, it will allow us to increase the reproductive efficiency of agriculturally important species. Secondly, understanding how to better handle mammalian embryos in vitro will benefit the biomedical community as a resource to generate animal models for human diseases. While the scientific community has gained tremendous insight into the mechanisms of many human diseases through the use of transgenic and knock-out mice, much more sophisticated models, perhaps using animals that are more 'physiologically relevant', may be found in genetically modified livestock species, like the pig.
Current projects in the lab are aimed at examining the how specific epigenetic modifications are mediated in the early embryo (e.g., histone methylation) and the mechanisms by which specific chromatin-interacting factors access the nucleus during development.
Jean Chmielewski
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Specific Disease(s)
- Diseases resulting from pathogenic bacteria, including Mycobacterium tuberculosis, MRSA, VRSA, Salmonella, Shigella, Listeria, Brucella, Acinetobacter baumannii, and others
- Bacteria biofilms
- HIV reservoirs
- Drug-resistant malaria
Molecular/Cellular Target(s)
- Intracellular pathogenic bacteria with an emphasis on subcellular localizations within mammalian cells
- HIV reservoirs in the brain
- Drug resistant P. falciparum with an emphasis on the chloroquine-resistance transporter (PfCRT)
Research Interest and Expertise
- We have expertise in the design and synthesis of novel non-membrane lytic antibacterial peptides that penetrate mammalian cells and their reversible conjugates with other antimicrobial agents.
- We have developed potent Trojan horse inhibitors of multidrug resistance transporters present at the blood-brain-barrier that revert to therapeutic agents within mammalian cells. This strategy can be used to improve the uptake of therapies (antiviral and anti-cancer) into the brain for HIV reservoir and tumor eradication.
- We have designed novel inhibitors of PfCRT within P. falciparum that effectively reverses drug resistance in cell culture with anti-malaria activity and show efficacy in a mouse model.
Daniel Hogan
Veterinary Clinical Sciences
Category of Research
- X Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Specific Disease(s)
Cardiac, vascular, thrombosis, valvular
Molecular/Cellular Target(s)
TGF-B and valvulopathy
Research Interest and Expertise
Our research focuses on cardiovascular therapeutics, including heart failure and antithrombotics. We have expertise in veterinary clinical trials, pre-clinical animal trials and animal modeling.
Daisuke Kihara
Biological Sciences/Computer Science
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Richard Kuhn
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Yuk Fai Leung
College of Science
Biological Sciences
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- X In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Specific Disease(s)
Retinal degeneration
Molecular/Cellular Target(s)
Retinas, photoreceptors, retinal ganglion cells
Research Interest and Expertise
Retinal degeneration is a group of inherited eye diseases including retinitis pigmentosa and age-related macular degeneration that impair our vision. They are incurable, even though much has been learned about the molecular basis of these diseases. To expedite discovery of new drugs for these diseases, we study zebrafish retinal-degeneration models.
We focus on two research directions:
- Disease-causing gene network for retinal degeneration
- Drug discovery for retinal degeneration.
Please visit our lab website for further information.
Zoltan Nagy
College of Engineering
Chemical Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Professor Nagy’s research is characterized by the development and application of process systems engineering approaches and tools for engineered product design and optimal process operation, with applications in pharmaceutical, fine chemical, biotechnology, food and agrochemical industries. Our research combines modeling, optimization and advanced control approaches with experimental investigations using modern measurement techniques, with the generic aim to develop theoretically founded, practical methodologies for complex processes with quantifiable system performance improvements that can be supported in an industrial environment.
Rodolfo Pinal
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Dr. Pinal’s research interests include: solution chemistry, solubility and solubilization techniques, mixtures, polymer-based composites as means of control of product performance (mechanical strength, dissolution, release rate and bioavailability) of bioactive compounds, antiplasticization, and molecular relaxation in amorphous organic materials.
Riyi Shi
College of Veterinary Medicine
Basic Medical Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- Other
Research Interest and Expertise
Our research contributions includes originating the use of double sucrose gap technique for recording action potential conduction, establishing the methods of neuronal membrane resealing by polyethelyne glycol (PEG), and identifying acrolein as a key pathological factor in spinal cord injury and multiple sclerosis. His research interests also include using nanotechnology to improve drug delivery to nervous tissue and incorporating biomedical engineering principles to enhance neuronal repair and diagnosis. This includes designing innovative scaffolds to enhance neuronal regeneration and using bioadhesives for neuronal tissue repair.
Daniel Smith
College of Pharmacy
Industrial And Molecular Pharmaceutics
Category of Research
- Diagnostics
- X Target Discovery & Characterization
- X Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
The Center for Paralysis Research and Department of Industrial and Physical Pharmacy focus on neurological trauma, endeavoring to discover and develop drugs for the treatment of spinal cord injuries.
Kevin Sowinski
College of Pharmacy
Department Of Pharmacy Practice
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Sex-related differences in drug pharmacokinetics and response; Impact of renal disease and dialysis on drug pharmacokinetics and response; Mathematical modeling of pharmacokinetic and pharmacodynamics; Cardiovascular pharmacokinetics and pharmacodynamics.
John Tesmer
Biological Sciences
Category of Research
- Diagnostics
- Target Discovery & Characterization
- X Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- X Neurological Disorder/Trauma
- X Other
James Tisdale
College of Pharmacy
Department Of Pharmacy Practice
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
My research interests are in the area of cardiovascular pharmacotherapy, focusing on: mechanisms, risk factors, and management of drug-induced arrhythmias, and drug therapy for prevention and treatment of atrial fibrillation.
Mohit Verma
Agricultural And Biological Engineering
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- X Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other
Jonathan Wilker
College of Science
Chemistry
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- X Other
Research Interest and Expertise
Our laboratory is working to understand how such biological materials function, design synthetic mimics, and develop applications for these materials.
Yuan Yao
Department Of Food Science
Category of Research
- Diagnostics
- Target Discovery & Characterization
- Synthesis/Optimization
- X Delivery/Formulations
- In Vivo Disease Models
- ADME/DMPK/Tox
- X Other
General Disease Area
- X Cancer
- Diabetes/Obesity/Metabolic Disease
- Immunology/Inflammatory/Infectious Disease
- Neurological Disorder/Trauma
- Other

- OFFICE OF THE EXECUTIVE VICE PRESIDENT
FOR RESEARCH AND PARTNERSHIPS - Hovde Hall
- 610 Purdue Mall
- West Lafayette, IN 47907-2040
- EVPRP@purdue.edu
- Phone: 765-494-9806
- Fax: 765-496-7474
- Updated April 2017